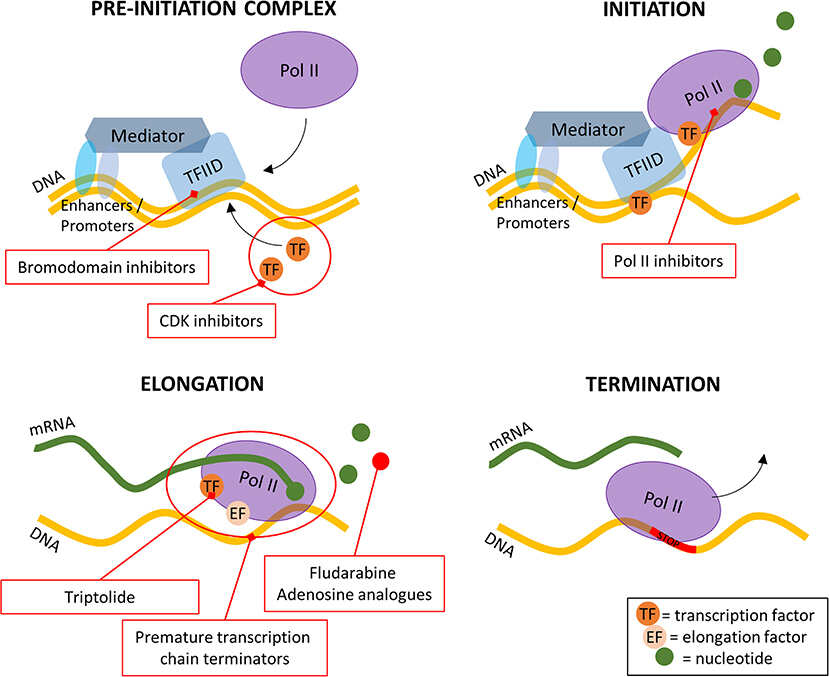
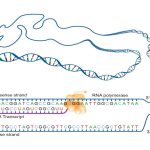
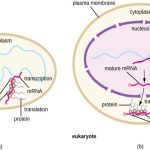
Bacteria, like mammalian cells, must synthesize proteins for self-maintenance and replication. DNA serves as the “instruction manual;” it provides the information necessary for protein synthesis. The first step in this process is transcription, the synthesis of a single-stranded ribonucleic acid (RNA) from the DNA template catalyzed by RNA polymerase The function of the newly synthesized RNA is translation.
In the process of translation, RNA serves three functions: (1) as messenger RNA (mRNA), it tells ribosomes which proteins to synthesize; (2) as transfer RNA (tRNA), it transports specific amino acids called for by mRNA codons from the cytoplasm to ribosomes; and (3) as ribosomal RNA (rRNA), it ensures that the amino acid carried by the charged tRNA is the one called for by the corresponding mRNA codon
Protein synthesis is initiated when the mRNA joins with the 30S ribosomal subunit and tRNA-linked formyl methionine (fMet).As the first amino acid encoded by every bacterial mRNA, fMet binds the initiation codon on the mRNA Next, the 30S-fMet-tRNA complex joins with the 50S ribosomal subunit to form the complete initiation complex, i.e., the 70S ribosomal unit, which contains two binding sites, an aminoacyl or A-site and a peptidyl or P-site (Figure 8).
Figure 8. Formation of the 70S Ribosomal Initiation Complex.
Modified from Ryou M, Coen DM. Pharmacology of bacterial infections: DNA replication, transcription, and translation. In Golan DE, Tashjian, Jr. AH, Armstrong EJ, Armstrong AW. Ed. Principles of pharmacology. The pathophysiologic basis of drug therapy. 2nd ed. 2008. Wolters Kluwer/Lippincott Williams & Wilkins. Baltimore, MD.
The P-site initially is occupied by the fMet-tRNA complex. As the next charged tRNA binds to the 70S ribosomal unit, but before it is allowed to enter the unoccupied A-site, the rRNA must confirm that the charged tRNA carries the specific amino acid called for by the mRNA codon. If access is allowed, the rRNA catalyzes the formation of a peptide bond between the carboxy-terminal of the fMet residing in the P-site and the new amino acid occupying the A-site (Figure 9).
Figure 9. The Process of Protein Synthesis.
Modified from Ryou M, Coen DM. Pharmacology of bacterial infections: DNA replication, transcription, and translation. In Golan DE, Tashjian, Jr. AH, Armstrong EJ, Armstrong AW. Ed. Principles of pharmacology. The pathophysiologic basis of drug therapy. 2nd ed. 2008. Wolters Kluwer/Lippincott Williams & Wilkins. Baltimore, MD.
Once the peptide bond is formed, the tRNA originally linked to fMet is ejected from the P-site and the second tRNA located at the A-site, which is now linked to two amino acids, translocates to the unoccupied P-site (Figure 9). As the process repeats itself, a growing peptide chain emerges from the exit tunnel. Translation continues until a stop codon is encountered in the mRNA and the newly synthesized protein is released from the ribosome.
Tetracyclines
Tetracycline and its semi-synthetic derivatives (e.g., minocycline and doxycycline) bind to 30S ribosomal subunits and reversibly block the attachment of the charged tRNA to the aminoacryl or A-site.They have bacteriostatic activity against aerobic gram-positive and gram-negative organisms, but in vivo many strains have been shown to be resistant. Tetracyclines are not empirical options in the treatment of odontogenic infections.
It is also of note that tetracyclines are teratogenic. They produce higher rates of neuronal-tube defect, cleft palate, and multiple congenital abnormalities, e.g., neuronal-tube defect with cardiovascular malformation. Furthermore, tetracyclines induce enamel hypoplasia and discoloration of teeth. Before prescribing tetracycline during pregnancy and/or tooth development the benefits and risks must be considered.
Aminoglycosides
Aminoglycosides (e.g., gentamicin) bind to 30S ribosomal subunits and induce misreading of mRNA codons. They are bactericidal in susceptible organisms and are active against many aerobic and facultative gram-positive and gram-negative cocci and bacilli, but most species of streptococci and anaerobic gram-negative bacilli are resistant. Aminoglycosides do not have the requisite spectra to be considered empirical options in treating odontogenic infections.
Clindamycin
Clindamycin binds to 50S ribosomal subunits and blocks peptide bond formation between amino acids located in the P- and A-sites (Figure 8). It has excellent activity against gram-positive aerobes and anaerobes, as well as gram-negative anaerobes. Consequently, clindamycin has the requisite spectrum to be considered an empirical option in treating odontogenic infections.
Clindamycin is rapidly and almost completely absorbed after oral administration and reaches peak plasma concentration in about 45 minutes. It is widely distributed in body fluids and tissues (including bone). Clindamycin is extensively metabolized in the liver and its metabolites are excreted primarily by the kidneys.
Macrolides
Macrolides bind 50S ribosomal subunits and block translocation and peptide movement through the exit tunnel.They are bacteriostatic in susceptible organisms and are active against aerobic gram-positive cocci and gram-negative bacilli, but anaerobic gram-negative organisms are resistant. Azithromycin has an extended spectrum that includes some anaerobic gram-positive cocci and gram-negative bacilli and may be considered an empirical option in treating odontogenic infections.
Azithromycin is rapidly absorbed after oral administration. When administered with food, however, its rate and extent of absorption is reduced by about 50%. The drug is widely distributed throughout the body, accumulating in high concentration within cells resulting in higher tissue than plasma concentrations. Azithromycin is metabolized minimally and is principally eliminated as unchanged drug via the liver.