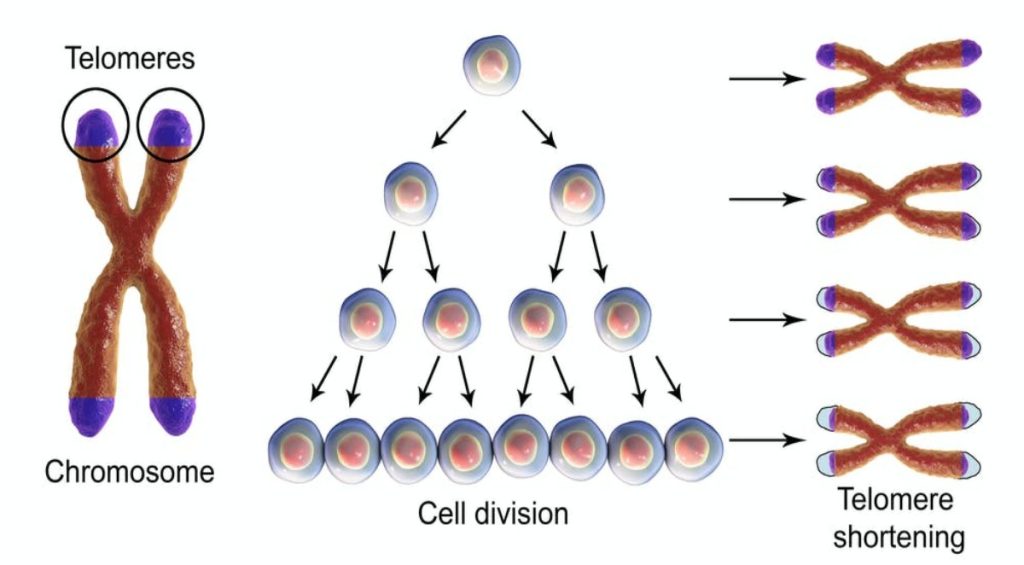
The telomeres are special structures on the chromosome ends that are essential for providing protection from enzymatic end-degradation and maintaining chromosomal and genomic stability. This is the reason why adequate telomere structure (including the presence of telomere-binding proteins) remains pivotal for avoiding cellular dysfunction.
Since their discovery in the mid-1980s, biology of telomeres attracted significant interest from the scientific community due to their aforementioned importance. There is a consensus that their length is reduced with age, smoking and stress, and that shorter telomeres can impair health.
General telomere structure
Telomeres are composed of a DNA component characterized by noncoding repetitive sequences rich in guanine (G) and multiple protein components. Repeat sequences can vary from one species to the other, with hexanucleotide repeat TTAGGG being characteristic for humans and other vertebrates.
The extreme ends of every telomere are not blunt, as the 3’ single-stand overhang comprises 200 nucleotides and loops back with some of the double-stranded telomeric DNA to make a telomere loop known as “T loop”. This event plays a protective role as it sequesters the overhang terminal inside the double strand.
The acquisition of telomere repeats was a cornerstone event in the evolution of eukaryotic nucleus, due to their fundamental roles in chromosome organization and stability of the genome. Telomeres are considered as localized structures at the chromosomal ends, they can also be found at internal positions.
A myriad of proteins are directly or indirectly associated with telomeric DNA. Some of these proteins (most notably TIN2, TRF1, TRF2, TPP1, and POT1) are found in telomeres at any time, even though there is a highly dynamic exchange between proteins that are telomere-bound and unbound.
The telomerase enzyme, which is a ribonucleoprotein with reverse transcriptase activity, is composed of two main parts – a telomere RNA component and a telomere reverse transcriptase. Although there is practically no activity of the telomerase in somatic cells, a low level is present in mitotically active cells.
Roles of telomeres and telomerase
The properties of the eukaryotic telomeres are usually identified as the “capping function”, with a principal mission to protect chromosome ends from DNA degradation, DNA repair mechanism and fusion with other chromosomal ends. Their length serves as an intrinsic biological clock that regulates the life span of the cell, i.e. they provide limits on the number of replications a cell can go through.
Uncapped telomeres are able to activate the DNA damage response and cause end-to-end fusions, resulting in chromosomal instability, cellular senescence and apoptosis (programmed cell death). Telomere repeats are lost with each round of cell replication by a plethora of different mechanisms, and most somatic cells express insufficient telomerase to compensate for the loss of telomere repeats.
The well-established function of telomerase is the elongation of telomeres, which enable cells to increase their replicative capacity (sometimes even indefinitely). However, low expression of telomerase (for example, in some normal fibroblasts) cannot maintain telomere length, but plays a role in maintaining chromosomal structure during each S phase of the cell cycle.
Telomerase also shows important roles in stem cell proliferation, as well as reprogramming of induced pluripotent stem cells. The mechanism of these telomerase functions is still not completely clear, as it may or may not be linked to maintenance of telomere length.