In order to synthesize a peptide from its component amino acids, two obstacles must be overcome. The first of these is statistical in nature, and is illustrated by considering the dipeptide Ala-Gly as a proposed target. If we ignore the chemistry involved, a mixture of equal molar amounts of alanine and glycine would generate four different dipeptides. These are: Ala-Ala, Gly-Gly, Ala-Gly & Gly-Ala. In the case of tripeptides, the number of possible products from these two amino acids rises to eight. Clearly, some kind of selectivity must be exercised if complex mixtures are to be avoided.
The second difficulty arises from the fact that carboxylic acids and 1º or 2º-amines do not form amide bonds on mixing, but will generally react by proton transfer to give salts (the intermolecular equivalent of zwitterion formation).
From the perspective of an organic chemist, peptide synthesis requires selective acylation of a free amine. To accomplish the desired amide bond formation, we must first deactivate all extraneous amine functions so they do not compete for the acylation reagent. Then we must selectively activate the designated carboxyl function so that it will acylate the one remaining free amine. Fortunately, chemical reactions that permit us to accomplish these selections are well known.
First, the basicity and nucleophilicity of amines are substantially reduced by amide formation. Consequently, the acylation of amino acids by treatment with acyl chlorides or anhydrides at pH > 10, as described earlier, serves to protect their amino groups from further reaction.
Second, acyl halide or anhydride-like activation of a specific carboxyl reactant must occur as a prelude to peptide (amide) bond formation. This is possible, provided competing reactions involving other carboxyl functions that might be present are precluded by preliminary ester formation. Remember, esters are weaker acylating reagents than either anhydrides or acyl halides, as noted earlier.
Finally, dicyclohexylcarbodiimide (DCC) effects the dehydration of a carboxylic acid and amine mixture to the corresponding amide under relatively mild conditions. The structure of this reagent and the mechanism of its action have been described. Its application to peptide synthesis will become apparent in the following discussion.
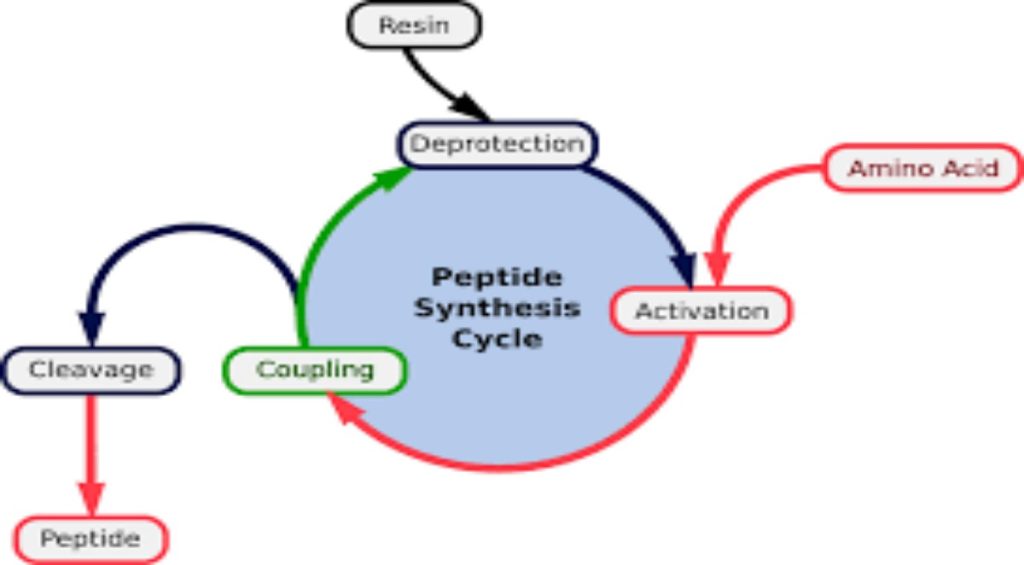
The strategy for peptide synthesis, as outlined here, should now be apparent. The following example shows a selective synthesis of the dipeptide Ala-Gly.

An important issue remains to be addressed. Since the N-protective group is an amide, removal of this function might require conditions that would also cleave the just formed peptide bond. Furthermore, the harsh conditions often required for amide hydrolysis might cause extensive racemization of the amino acids in the resulting peptide. This problem strikes at the heart of our strategy, so it is important to give careful thought to the design of specific N-protective groups. In particular, three qualities are desired:
- The protective amide should be easy to attach to amino acids.
- The protected amino group should not react under peptide forming conditions.
- The protective amide group should be easy to remove under mild conditions.
A number of protective groups that satisfy these conditions have been devised; and two of the most widely used, carbobenzoxy (Cbz) and t-butoxycarbonyl (BOC or t-BOC), are described here.


The reagents for introducing these N-protective groups are the acyl chlorides or anhydrides shown in the left portion of the above diagram. Reaction with a free amine function of an amino acid occurs rapidly to give the “protected” amino acid derivative shown in the center. This can then be used to form a peptide (amide) bond to a second amino acid. Once the desired peptide bond is created the protective group can be removed under relatively mild non-hydrolytic conditions. Equations showing the protective group removal will be displayed above by are shown above. Cleavage of the reactive benzyl or tert-butyl groups generates a common carbamic acid intermediate (HOCO-NHR) which spontaneously loses carbon dioxide, giving the corresponding amine. If the methyl ester at the C-terminus is left in place, this sequence of reactions may be repeated, using a different N-protected amino acid as the acylating reagent. Removal of the protective groups would then yield a specific tripeptide, determined by the nature of the reactants and order of the reactions.
Comments are closed