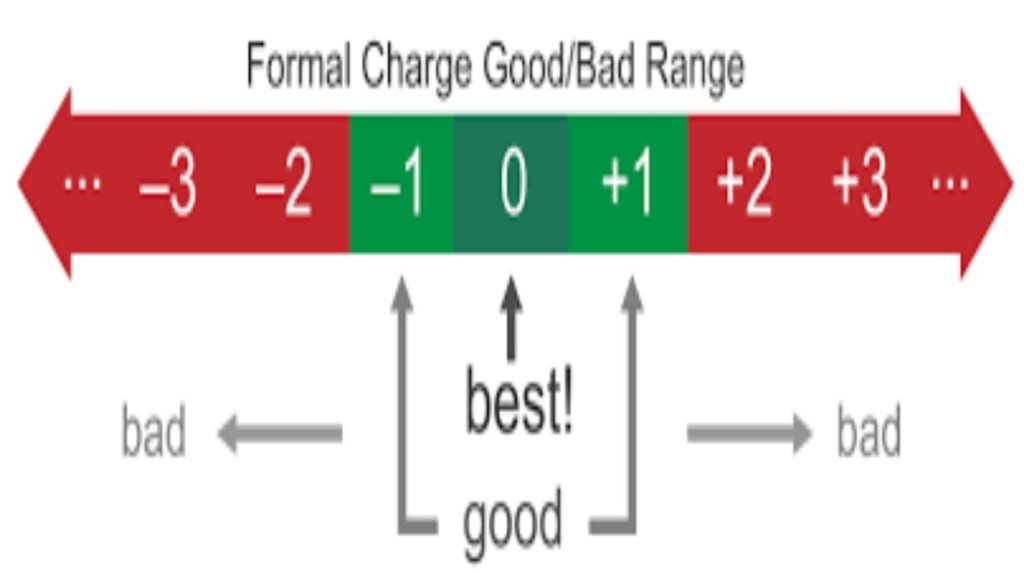
Formal charges in organic chemistry is, perhaps, one of the most fundamental bookkeeping devices which is often misunderstood or neglected by students.
Why Formal Charges are Important in Organic Chemistry?
Knowing formal charges can help us understand the reactivity patterns in reactions, find reactive centers, and make sense out of electron flow in the mechanisms. For instance, negatively charged species tend to be the sources of electron density in reactions, while the positively charged species—accept those electrons.
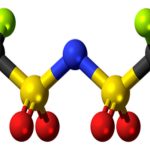
Here is a couple of examples of molecules with formal charges:

Examples of molecules with formal charges.
The top species has a negative charge. We call such species anions. Since it has a negative charge, it means that it has an excess of electron density. Thus, it is likely to be the source of those electrons in a reaction. Sources of electron density in an organic reaction are acting as nucleophiles or bases.
The second species bears a positive charge, thus it is a cationic species. In a reaction, a cationic species will be an acceptor of electrons acting as an acid (either Brønsted or Lewis) or as an electrophile.
It’s important to keep in mind that a formal charge is not the same thing as an actual charge! I’ll talk about it a little later in this post.
How to Calculate a Formal Charge
The “official” way is to subtract 1/2 bonding electrons and nonbonding electrons from possible valence electrons that an atom can have. In other words:
 Formula for calculating formal charge
Formula for calculating formal charge
What are all those terms?
Bonding electrons are those that make up bonds. Each bond contains 2 electrons. So, 1/2 of bonding electrons equals to the number of bonds and atom has.
Nonbonding electrons are those that are not participating in any bonding. In other words, nonbonding electrons are the spare electrons (usually electron pairs) on an atom. Using the examples from above we have:

Examples of formal charge calculations
The definitions and the “official” method looks a little ugly. As a professional chemist I can talk all day about the “official rules” and “proper names” and bore you to death. Instead, I’d rather you use a simple “trick” that always works and, essentially, is the same thing. The trick is:
Formal charge = Valence Electrons – Sticks – Dots
The number of valence electrons equals to the element’s group (column) in the periodic table. This way, carbon has 4, oxygen has 6, and hydrogen has 1 valence electrons. The bonds and the spare electrons will be indicated (or can be easily found from) the molecule’s Lewis structure. So, for as long as you have a complete Lewis structure and periodic table handy, you can quickly find the formal charge of any atom in a molecule.