Researchers at the Cleveland Clinic announced in a press release on Tuesday, October 26, that they have the green light from the U.S. Food and Drug Administration to start the first phase of a clinical trial that aims to prevent triple-negative breast cancer.
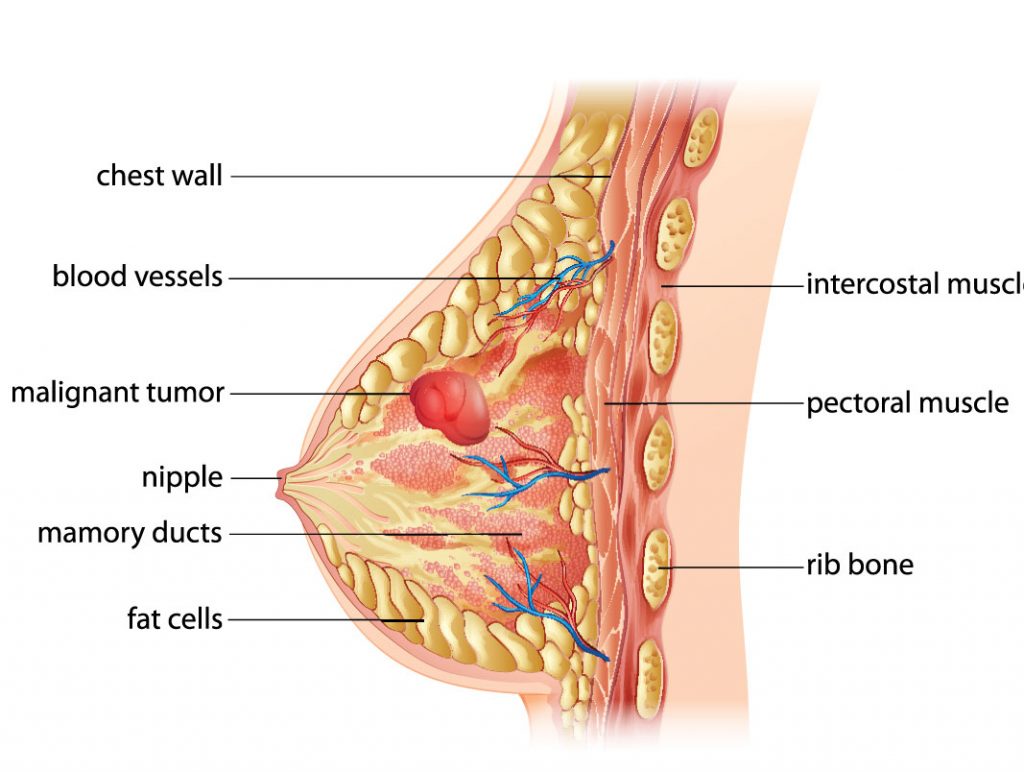
This form of breast cancer is the most aggressive and deadly form of the disease. It also grows and spreads faster, and has a higher chance of recurrence. It accounts for 10 to 15 percent of all breast cancers, is more common in women under 40, African-American women, and women who have the BRCA1 mutation, reports the American Cancer Society.
To date, there are limited treatment options, and a worse prognosis. So it’s very easy to see why researchers at the Cleveland Clinic are pushing their clinical trial forward.
Funded by the U.S. Department of Defense, the study involves 18 to 24 participants. All patients have completed treatment for early-stage triple-negative breast cancer within the last three years, currently have no tumor, and are at a higher risk of recurrence.
Throughout the study, the patients will be administered three vaccinations every two weeks, and their results and side-effects will be closely monitored. The end of the study is set for September 2022, per the press release.
The point of this first phase is to determine the maximum dose of the vaccine in patients with early-onset triple-negative breast cancer, and to evaluate its effects on the participants. The subsequent trials hope to then “determine the effectiveness of the vaccine against this highly aggressive type of breast cancer,” stated G. Thomas Budd, the principal investigator of the study.
“The long-term objective of this research is to determine if this vaccine can prevent breast cancer before it occurs, particularly the more aggressive forms of this disease that predominate in high-risk women,” added Vincent Tuohy, the primary inventor of the vaccine.
The mechanism of the vaccine
The vaccine targets a lactation protein no longer found in post-lactation in normal, aging tissues, but that is present in most triple-negative breast cancers: α-lactalbumin. Boosting the immune system against this “retired” protein should hopefully pre-emptively protect people at risk of upcoming breast tumors that express α-lactalbumin.
Aside from vastly improving and prolonging the lives of triple-negative breast cancer patients, this vaccine strategy could also be used against other tumors, explains the Cleveland Clinic team.
Hopefully, it will end up joining the other types of treatments or preventative measures, such as bee venom or the ErSO drug.
As Tuohy explains, “If successful, these vaccines have the potential to transform the way we control adult-onset cancers and enhance life expectancy in a manner similar to the impact that the childhood vaccination program has had.”