Previously, constitutional isomers were defined as molecules that had the same molecular formula, but different atom connectivity. In this section, a new class of isomers, stereoisomers, will be introduced. Stereoisomers are molecules that have the same molecular formula, the same atom connectivity, but they differ in the relative spatial orientation of the atoms.
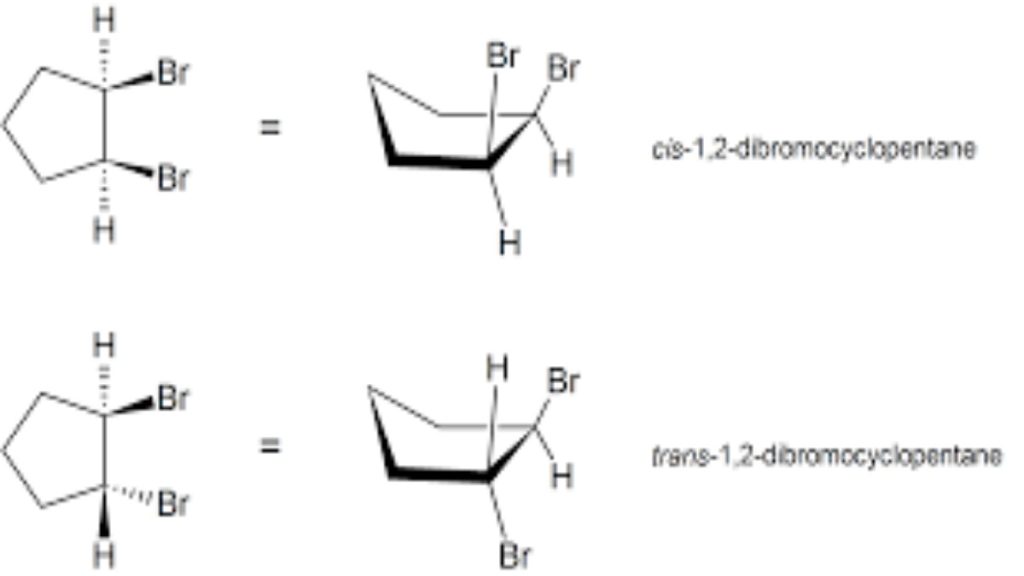
Di-substituted cycloalkanes are one class of molecules that exhibit stereoisomerism. 1,2-dibromocyclopentane can exist as two different stereoisomers: cis-1,2-dibromocyclopentane and trans-1,2-dibromocyclopentane. The cis-1,2-dibromocyclopentane and trans-1,2-dibromocyclopentane stereoisomers of 1,2-dibromocyclopentane are shown below. Both molecules have the same molecular formula and the same atom connectivity. They differ only in the relative spatial orientation of the two bromines on the ring. In cis-1,2-dibromocyclopentane, both bromine atoms are on the same “face” of the cyclopentane ring, while in trans-1,2-dibromocyclopentane, the two bromines are on opposite faces of the ring. Stereoisomers require an additional nomenclature prefix be added to the IUPAC name in order to indicate their spatial orientation. Di-substituted cycloalkane stereoisomers are designated by the nomenclature prefixes cis (Latin, meaning on this side) and trans (Latin, meaning across).

By convention, chemists use heavy, wedge-shaped bonds to indicate a substituent located above the average plane of the ring (coming out of the page), a dashed line for bonds to atoms or groups located below the ring (going back into the page), and solid lines for bonds in the plane of the page.

In general, if any two sp3 carbons in a ring have two different substituent groups (not counting other ring atoms) cis/trans stereoisomerism is possible. However, the cis/trans designations are not used if both groups are on the same carbon. For example, the chlorine and the methyl group are on the same carbon in 1-chloro-1-methylcyclohexane and the trans prefix should not be used.

If more than two ring carbons have substituents, the stereochemical notation distinguishing the various isomers becomes more complex and the prefixes cis and trans cannot be used to formally name the molecule. However, the relationship of any two substituents can be informally described using cis or trans. For example, in the tri-substituted cyclohexane below, the methyl group is cis to the ethyl group, and also trans to the chlorine. However, the entire molecule cannot be designated as either a cis or trans isomer. Later sections will describe how to name these more complex molecules (5.5: Sequence Rules for Specifying Configuration)