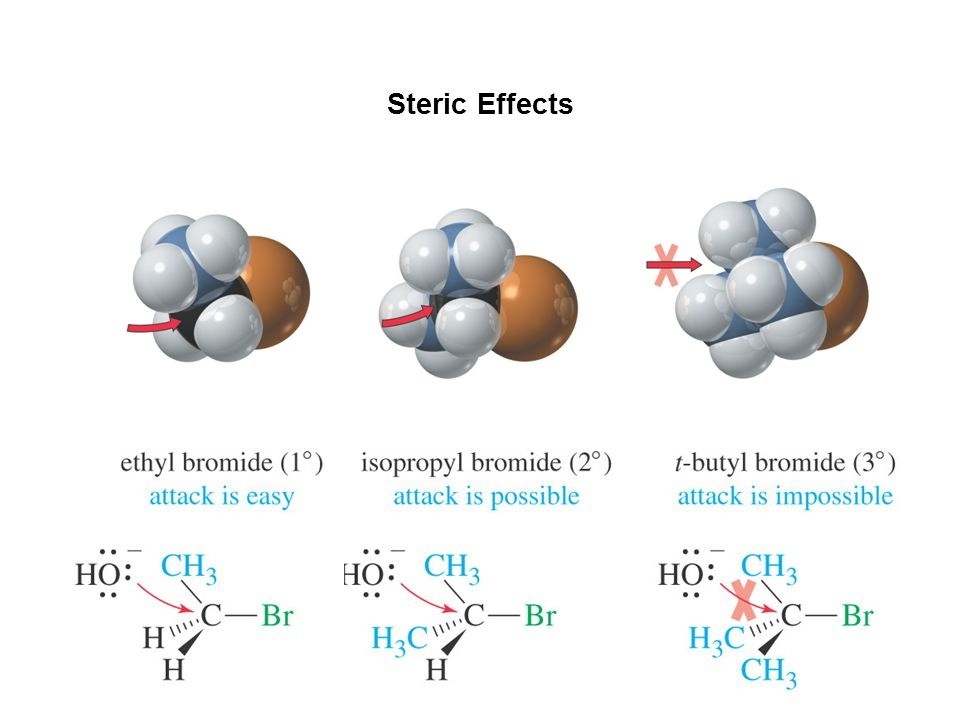
In general, the steric effect refers from the fact that the atoms composing molecules occupy some degree of space, and when atoms come too close together there’s a rise in the energy of the molecule due to the atoms being forced to occupy the same physical space.
It turns out that steric effects can have a dramatic effect on the observed or preferred shape of a molecule and in some cases even its chemical reactivity. In its very simplest form, steric effects can be thought of as a ‘crowding’ effect kind of like that crowded room of people we mentioned earlier.
Consider as an example the molecule known as tert-butanol. The three bulky methyl groups labeled in blue are what makes this molecule so sterically crowded. Both the central carbon atom and the alcohol group are what we would call ‘sterically shielded’ due to the presence of the large methyl groups.
 |
| Tert-butanol is an example of a very sterically crowded molecule |
Example: Grignard Reaction
Now that we’re a bit more familiar with the steric effect, let’s take a look at some example compounds to get a better feel for it.
Sometimes if two molecules are very sterically demanding (take up lots of space) a chemical reaction that you think would be trivial actually won’t occur at all. An example would be a reaction called a Grignard addition, which occurs between a ketone and a magnesium-bromide reagent, called the Grignard reagent.
In this specific Grignard reaction, no chemical reaction occurs at all because of steric effects. Both the ketone and the Grignard reagent are so crowded due to the bulky methyl groups that no product is observed when the two reactions are mixed.
 |
| This Grignard reaction does not work due to steric effects |