Introduction
It has been demonstrated that water adds rapidly to the carbonyl function of aldehydes and ketones to form geminal-diol. In a similar reaction alcohols add reversibly to aldehydes and ketones to form hemiacetals (hemi, Greek, half). This reaction can continue by adding another alcohol to form an acetal. Hemiacetals and acetals are important functional groups because they appear in sugars.
To achieve effective hemiacetal or acetal formation, two additional features must be implemented. First, an acid catalyst must be used because alcohol is a weak nucleophile; and second, the water produced with the acetal must be removed from the reaction by a process such as a molecular sieves or a Dean-Stark trap. The latter is important, since acetal formation is reversible. Indeed, once pure hemiacetal or acetals are obtained they may be hydrolyzed back to their starting components by treatment with aqueous acid and an excess of water.
Formation of Hemiacetals

FORMATION OF HEMIACETALS

HEMIACETAL REVERSIBILITY

Formation of Acetals
Acetals are geminal-diether derivatives of aldehydes or ketones, formed by reaction with two equivalents (or an excess amount) of an alcohol and elimination of water. Ketone derivatives of this kind were once called ketals, but modern usage has dropped that term. It is important to note that a hemiacetal is formed as an intermediate during the formation of an acetal.

FORMATION OF ACETALS

ACETAL REVERSIBILITY

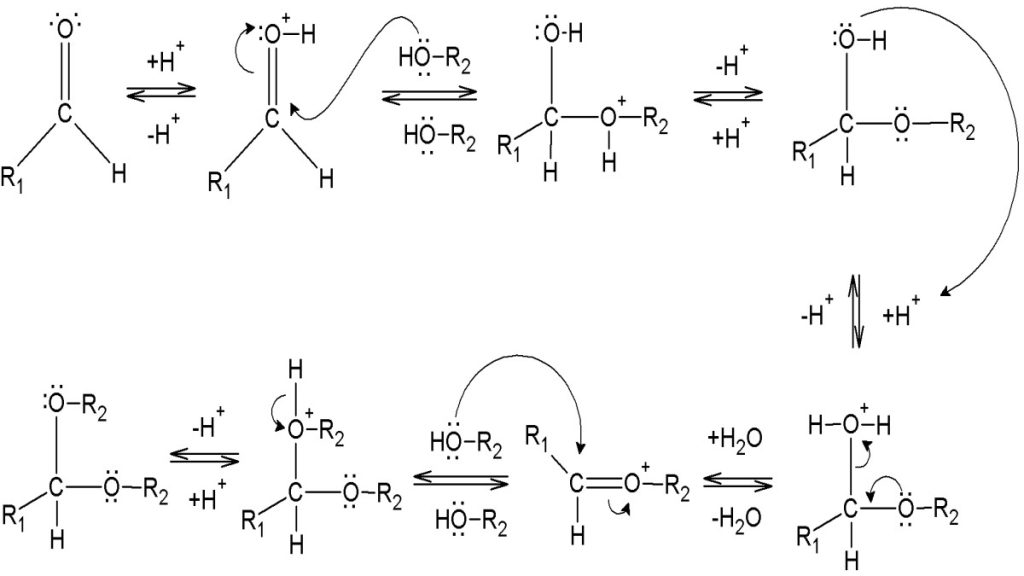
Mechanism for Hemiacetal and Acetal Formation
The mechanism shown here applies to both acetal and hemiacetal formation
1) Protonation of the carbonyl

2) Nucleophilic attack by the alcohol

3) Deprotonation to form a hemiacetal

4) Protonation of the alcohol

5) Removal of water

6) Nucleophilic attack by the alcohol

7) Deprotonation by water

Acetals as Protecting Groups
The importance of acetals as carbonyl derivatives lies chiefly in their stability and lack of reactivity in neutral to strongly basic environments. As long as they are not treated by acids, especially aqueous acid, acetals exhibit all the lack of reactivity associated with ethers in general. Among the most useful and characteristic reactions of aldehydes and ketones is their reactivity toward strongly nucleophilic (and basic) metallo-hydride, alkyl and aryl reagents. If the carbonyl functional group is converted to an acetal these powerful reagents have no effect; thus, acetals are excellent protective groups, when these irreversible addition reactions must be prevented.
In the following example we would like a Grignard reagent to react with the ester and not the ketone. This cannot be done without a protecting group because Grignard reagents react with esters and ketones.