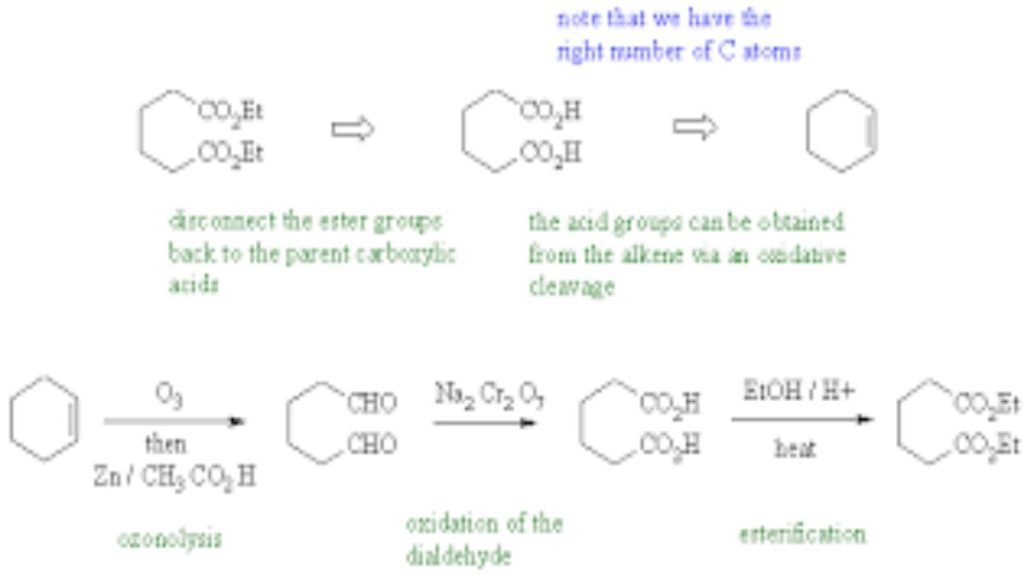
we introduced the enolates of aldehydes and ketones (review) and looked at their reactions as C nucleophiles (review). These enolates were formed by treating the aldehyde or ketone with a suitable base :
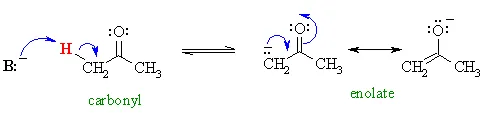
Now we will investigate another group of carbonyl containing compounds, the esters, which behave in a very similar fashion…..
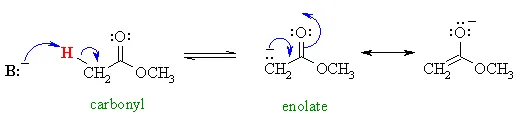
When preparing ester enolates, the base is normally chosen to match the alcohol portion of the ester…
So if you have:
a methyl ester, RCOOCH3 you use methoxide, –OCH3, or,
an ethyl ester, RCOOCH2CH3 you use ethoxide, –OCH2CH3
This avoids problems caused by transesterification (conversion of one ester into another)……
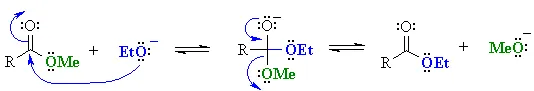
or hydrolysis……
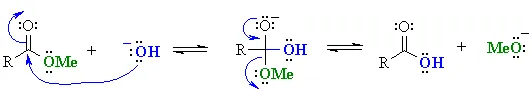
Note that in both cases the problem arises because the base reacts as a “nucleophile“