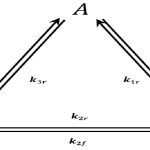
What exactly is a reaction intermediate? A reaction intermediate is formed from the reactants in a chemical reaction, and reacts further to produce the products observed after the reaction is complete. Let’s say you were going to be driving from New Mexico over to California. In order to get to California, you decide to drive through Arizona and Nevada for your route of travel. In terms of a chemical reaction, Arizona and Nevada would represent reaction intermediates because they certainly aren’t where you’re ultimately going, but you do have to go through them along the way to getting to California.
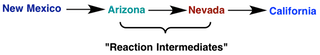 |
| Using a road trip as an analogy to reaction intermediates |
The most important thing to remember about a reaction intermediate is that even though it may not be shown in the overall chemical equation, it’s still a part of the process or a ‘stop’ in going from reactants to products.
Types of Reaction Intermediates
Now that we have a better idea of what a reaction intermediate is, let’s take a look at some specific examples. Keep in mind that these certainly aren’t exhaustive as all the types that can exist, but are the most common in the organic chemistry (the sub-discipline of chemistry that deals with carbon and hydrogen-based compounds) arena.
Carbanions
In organic chemistry, a carbanion (referred to as a carbonium ion in some texts) is a reaction intermediate in which there is a negative one charge located on a carbon atom. Carbanions are formed by treating an organic compound with a VERY strong base. Consider as an example the reaction of butane with a base. When the base pulls off a hydrogen atom from butane, a carbanion is formed.
 |
| Reaction of butane with a base to form a carbanion intermediate |
Carbanions are extremely reactive, and once they are formed in a chemical reaction they typically don’t last very long. Usually they go on further to react with a positive species in the reaction to form the final product of the reaction. This should make sense to us because we are forming a negatively charged intermediate, meaning it will be attracted to something else that has some sort of positive character to it.
Free Radicals
Another common class of reaction intermediates are free radicals. Free radicals contain a single, unpaired electron. They result when a covalent bond (a bond composed of two electrons) is broken and each atom takes one electron from the bond. For example, when a carbon-hydrogen bond in methane is broken, one of the electrons from the bond goes to carbon and the other electron goes to hydrogen. Notice that when we are representing free radicals, we use single dots on the atom in which the radical is located.
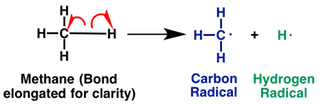 |
| Formation of a carbon radical and a hydrogen radical from methane |