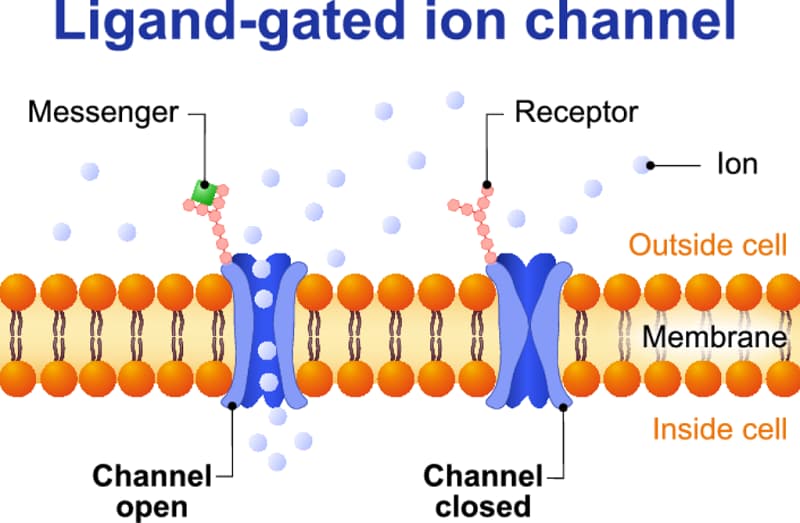
Ligand-gated ion channels (LGICs) are integral membrane proteins that contain a pore which allows the regulated flow of selected ions across the plasma membrane. Ion flux is passive and driven by the electrochemical gradient for the permeant ions. These channels are open, or gated, by the binding of a neurotransmitter to an orthosteric site(s) that triggers a conformational change that results in the conducting state. Modulation of gating can occur by the binding of endogenous, or exogenous, modulators to allosteric sites. LGICs mediate fast synaptic transmission, on a millisecond time scale, in the nervous system and at the somatic neuromuscular junction. Such transmission involves the release of a neurotransmitter from a pre-synaptic neurone and the subsequent activation of post-synaptically located receptors that mediate a rapid, phasic, electrical signal (the excitatory, or inhibitory, post-synaptic potential). However, in addition to their traditional role in phasic neurotransmission, it is now established that some LGICs mediate a tonic form of neuronal regulation that results from the activation of extra-synaptic receptors by ambient levels of neurotransmitter. The expression of some LGICs by non-excitable cells is suggestive of additional functions.
By convention, the LGICs comprise the excitatory, cation-selective, nicotinic acetylcholine, 5-HT3, ionotropic glutamate and P2X receptors and the inhibitory, anion-selective, GABAA and glycine receptors . The nicotinic acetylcholine, 5-HT3, GABAA and glycine receptors (and an additional zinc-activated channel) are pentameric structures and are frequently referred to as the Cys-loop receptors due to the presence of a defining loop of residues formed by a disulphide bond in the extracellular domain of their constituent subunits . However, the prokaryotic ancestors of these receptors contain no such loop and the term pentameric ligand-gated ion channel (pLGIC) is gaining acceptance in the literature . The ionotropic glutamate and P2X receptors are tetrameric and trimeric structures, respectively. Multiple genes encode the subunits of LGICs and the majority of these receptors are heteromultimers. Such combinational diversity results, within each class of LGIC, in a wide range of receptors with differing pharmacological and biophysical properties and varying patterns of expression within the nervous system and other tissues. The LGICs thus present attractive targets for new therapeutic agents with improved discrimination between receptor isoforms and a reduced propensity for off-target effects. The development of novel, faster screening techniques for compounds acting on LGICs will greatly aid in the development of such agents.