B Cell Activation
· Resting B cells become activated by antigen via the BCR and/or by microbiological side products (pathogen associated molecular patterns; PAMP) via their toll like receptors (TLR4, 7, 9 in mice) and start to proliferate.
· Protein antigens become internalized, digested and presented to T cells as peptides via MHCII.
· Cognate B cell / T cell interaction provides co-stimulation to B cells via CD40, which becomes activated on B cells via CD40 ligand (CD40L) expressed on T cells.
· T cells also provide cytokines to B cells that support their survival (IL-4), differentiation into plasma cells (IL-21) or class switch recombination.
· In mice, Th1 cytokines, such as IFNγ, typical for antiviral responses, elicit IgG2 isotypes, Th2 cytokines, such as IL-4, IL-5 and IL-13, typical for parasite infections, elicits IgG1 and IgE responses.
Precursors of plasma cells
· Resting B2 B cells (these are follicular (FO) and marginal zone (MZ) B cells) express membrane bound IgM, the B cell receptor (BCR) of the IgM type
· In mice and likely also in humans, another B cell population that is located primarily to the pleural cavities exists, the B1 B cells, a self-renewing population. B1 B cells secrete natural IgM in a T cell independent manner and have a limited repertoire. They differentiate rapidly into short lived plasma cells
· MZ B cells reside in the marginal zone that surrounds the follicles of secondary lymphoid organs and is directly connected to the vasculature. MZ B cells therefore respond to blood born antigens. They react more rapidly to PAMPs and differentiate rapidly into short lived plasma cells
· FO B cells express also membrane bound IgD
· FO B cells reside in the follicles of secondary lymphoid organs and are long lived. They are typically the B cells that interact with cognate T cells.
· Memory B cells express membrane bound Ig of the IgM, IgG or IgA type.
Activation of B cells
· Activation of B cells via the BCR and/or PAMPs elicits proliferation of B cells.
· PAMPs, for instance lipopolysaccharide, also elicit differentiation of B cells into short-lived plasma cells secreting low-affinity antibodies.
· MZ B cells are especially prone to rapidly differentiate into short-lived plasma cells but FO B cells can also differentiate into short- lived plasma cells.
· B cell differentiation into plasma cells is coupled to a certain number of cell divisions that are required to allow expression of transcription factors which terminate the B cell program and initiate the plasma cell program.
· T cell dependent activation of B cells supports the generation of memory B cells and long-lived plasma cells secreting high affinity antibodies. This process requires specific microanatomical structures in secondary lymphoid organs, the germinal centers, where class switch recombination and somatic hypermutation occur.
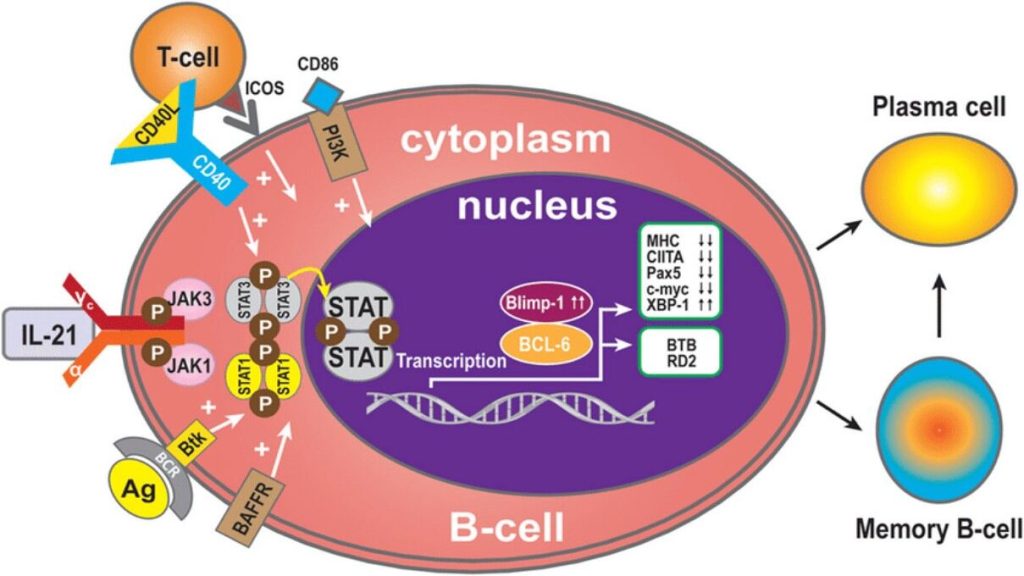
· The transcriptional network that is initiated in B cells in a proliferation dependent manner and fosters plasma cell differentiation is outlined in Fig. 1 (Taken from Nutt et al., Nature Reviews in Immunology, 2015).
· An essential transcription factor for plasma cell differentiation is Blimp-1. Many essential functions in plasma cells are under the control of Blimp-1.


The plasma cell and antibody secretion
· Early plasma cells still proliferate and are also called plasmablasts or antibody secreting cells (ASC).
· The term “antibody secreting plasma cell” is unambiguous and refers to the terminally differentiated, non-proliferating plasma cell that secretes high amounts of antibody. Plasma cells are much larger than resting or even proliferating B cells.
· In plasma cells and plasmablasts, the exons coding for the membrane domain of Ig molecules of various isotypes have been removed and replaced by exons coding for sequences allowing antibody secretion.
· Plasma cells have a strongly expanded endoplasmic reticulum and express high amounts of molecules involved in antibody folding and secretion.
· Plasma cells can secrete several thousands of antibodies per second.
· Long-lived plasma cells migrate back to the bone marrow where their survival is supported in niches. Stable antibody titers upon vaccination have been observed for 20 years and more for some immunogens.