Lactic acid was the first organic acid produced by fermentation process carried out in 1880. Today, it is manufactured both by chemical as well as fermentation methods. Chemically it is produced from lactonitrile, degradation of sugars, from carbon monoxide and acetaldehyde.
Chemical Structure of Lactic Acid:
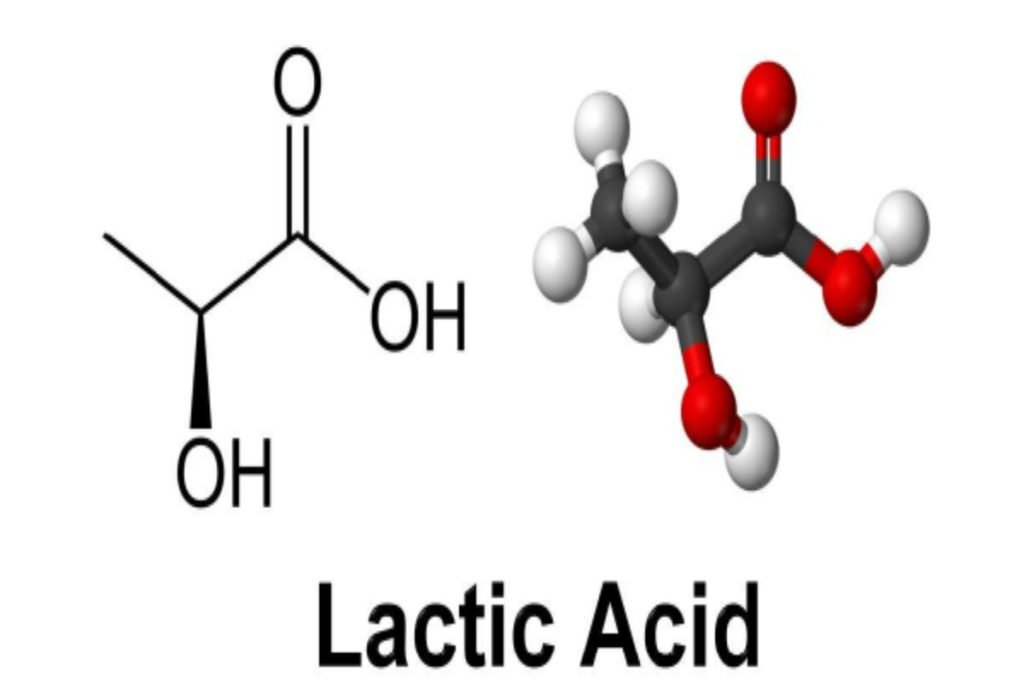
The structure of lactic acid is shown in Fig. 4.4.

It consists of an acid functional group (-COOH) and a hydroxyl group (-OH). Though it contains hydroxyl functional group, it exhibits more acidic properties. It contains an asymmetric carbon atom due to which lactic acid exists in two forms as L (+) lactic acid and D-lactic acid. L-lactic acid is useful as it can be metabolised by animal or human body, while the D- configuration is not metabolized and is largely excreted. However, in industrial fermentations racemic mixture of both D and L is obtained.
Biosynthesis of Lactic Acid:
The biosynthesis of lactic acid from glucose proceeds via glyceraldehyde-3- phosphate, 1, 3 diphosphoglycerate and pyruvate. The reducing power is produced during the oxidation of glyceraldehyde. Phosphate is transferred with NAD-dependent lactate dehydrogenase to pyruvate which reduces stereospecifically to L (+) or D (-) lactic acid (Fig. 4.5).

Fermentation Process of Lactic Acid:
Both homofermentators like Lactobacillus bulgaricus, L. plantarum, L. delbrueckii, Streptococcus lactis and Pediococcus sp. and heterofermenters like leuconostoc mesenteroides produces lactic acid. Characteristics of bacteria and fungi producing lactic acid both by batch and continuous fermentation, along with organisms employed, type of bioreactor, raw material used, lactic acid productivity are precised in table 4.3 and 4.4, respectively.


Different bacteria utilize different metabolic pathway for production of lactic acid (Fig. 4.6).

(i) Inoculum Production:
Microorganisms used in the lactic acid production are the species of Lactobacillus. Two types of lactic acid bacteria are recognized Heterofermentative and Homofermentative organisms. Heterofermentative organisms produce large number of byproducts and, therefore, not employed in commercial process. The homofermentative organisms produce very little number of byproducts and more lactic acid, therefore, these are employed in lactic acid fermentation (table 4.5).

The selection of species of Lactobacillus largely depends upon the nature of the carbon source being used in the fermentation. Lactobacillus delbrueckii and Lactobacillus leichmannii are employed when glucose is used as substrate, Lactobacillus bulgaricus when whey is used as substrate and Lactobacillus pentosus when sulfite waste liquor is used as substrate. These organisms are facultative rather than obligate anaerobes and therefore, bioreactors need not be run with complete oxygen exclusion.
Suspension of pure bacterial culture is prepared from the suitable high yielding strain of stock culture. The selected Lactobacillus culture is transferred to large vessel and the temperature is maintained at 45 to 50°C. Each stage of culture building requires 16-18 hours depending upon the volume of fermentation broth. About 5% inoculum volume is usually used in the fermentation process.
(ii) Preparation of Medium:
To satisfy the nutritional requirements of the microorganisms the medium should be prepared in such a way as to contain carbon source, nitrogen source, mineral source and source for growth factors. Glucose, maltose, lactose etc. or crude substrates such as corn starch, potato and rice, whey, molasses, sulfite waste liquor etc. are used as carbon source.
Pretreatment of starchy material by amylase or by sulphuric acid is necessary to bring about hydrolysis and to change complex starch into simple sugars like glucose and maltose. If sulfite waste liquor is used in the medium, it is essential to steam strip the sulfite to lyse to sulphur dioxide and also to treat it with alkali, to remove lysis during the pretreatment operation.
The sugar content in the medium is maintained at 5-12%. Though various ammonium salts can be used, ammonium hydrogen phosphate is usually employed as nitrogen source in the fermentation of lactic acid. About 0.25% of ammonium salts are added to the medium. Based on the requirement of the selected microorganism, minerals are added to the medium. Calcium carbonate is also added as a buffering agent to control pH.
Lactobacillus requires vitamins, specially vitamin B complexes for proper growth. These are added to the medium in the form of crude vegetable sources like the rootlets of germinating seeds of barley.
(iii) Fermentation Process:
Lactic acid production is carried in 25-125 klt fermenters. Lactic acid is quite corrosive, therefore, wooden fermenters are used commercially rather than metal fermenters. The pH of the fermentation broth is maintained at 5.5-6.5 by using calcium carbonate. Maintenance of pH is essential because it helps not only in the increase of fermentation rate but also in the increase of the yield. Maintenance of low pH values also helps in the elimination of bacterial contamination and also in the sterilization at low temperatures.
As more and more lactic acid is formed it may become toxic to the organism. Hence, neutralization of accumulated acid is done by the continuous addition of calcium hydroxide. Generally, the fermentation duration is 5-10 days. It can also be completed in 72 hours if 12 to 13% of glucose is used in the medium and pH is maintained at 6.3-6.5 by continuous neutralization of the acid formed. However, complete non supply of air is also not advisable because the bacteria employed are facultative aerobes.
The fermentation broth is gently agitated to keep the uniform distribution of nutrients and air. Though temperature is generally maintained at 45-50°C, maintenance of particular range of temperature depends to some extent on the type of organism employed. For example Lactobacillus delbrueckii and L.bulgaricus requires 45-50°C, while L.casei and L.pentosus requires 30°C.
Theoretically two molecules of lactic acid are produced from one molecule of glucose. Over 90% of this theoretical yield is actually attained in practice. The commercially produced lactic acid belongs to L (+) type.
(iv) Harvest and Recovery:
Technical or crude grade which is coloured and available with 22, 44, 50, 60 and 80% concentrations, while edible grade is straw coloured and is marketed 50 to 80% strength of colourless high purity lactic acid, the plastic grade marketed at 50 to 80% strengths and U.S.P. lactic acid is marketed at 85% strength. Other commercial preparations of lactic acid are calcium lactate, sodium lactate and copper lactate. The recovery method varies depending upon the grade of lactic acid required.
However, a general method of recovery process, described here, has the following steps:
1. The fermentation broth is filtered by using porcelain filters to separate the bacterium.
2. The filtrate is acidified with sulphuric acid to regenerate lactic acid, to precipitate calcium as calcium sulphate and is washed.
3. The washed filtrate is treated with activated carbon to remove organic impurities.
4. Repeated refining and evaporation steps are undertaken to get higher percentage of lactic acid to the extent of 50-60%.
5. It is then treated with ferrocyanide to remove heavy metals, if any, like copper.
6. It is finally purified by passing through ion exchange resins.
Lactic acid is also extracted and purified by some other methods such as:
1. Zinc salt of lactic acid is made which has low solubility and can be recovered,
2. Lactic acid is directly extracted with isopropyl ether by counter current method,
3. Methyl ester of lactic acid is prepared which is easily hydrolysed with boiling in water. Methyl alcohol is recovered,
4. High vacuum distillation of lactic acid.
Uses of Lactic Acid:
It has many uses. Some of them are described below:
1. It is a weak acid with good solvent properties. It is used as a preservative in food industry.
2. It readily polymerizes and, therefore, used in polymer industry.
3. It is also used to treat the fibers in the textile industries and laundry.
4. Calcium lactate is employed as baking powder and as a source of calcium in pharmaceutical industries.
5. Pure lactic acid is used in plastic industry.
6. Ethyl lactate is used in preparation of anti-inflammatory drugs.
7. It is used in manufacture of self-dissolving suture threads in surgery.
8. As a blood coagulant and dietary calcium source.
9. In the manufacture of cellophane resins, biodegradable plastics and some herbicides and pesticides.
10. It is used in food industry as acidulent as it has strong odour or flavour. Ethyl and butyl lactate are used as flavouring ingredients.