Nucleophiles are basically electron rich species which have the ability to donate electron pairs, as discussed earlier. Because of this electron pair donating tendency, all nucleophiles are Lewis Bases.
The word ‘nucleophile’ can be split into two parts, namely nucleus and philos. Philos is the Greek word for ‘love’. Therefore, nucleophiles can be thought of as Nucleus Loving species. These nucleophiles may have either a negative, or a neutral charge.
Some terminologies regarding nucleophiles are discussed below.
- The nucleophilic nature of a species describes the affinity of the species to the positively charged nucleus.
- Nucleophilicity is a word used to compare the nucleophilic character of different nucleophiles in question. It can also be called the nucleophile strength of a species.
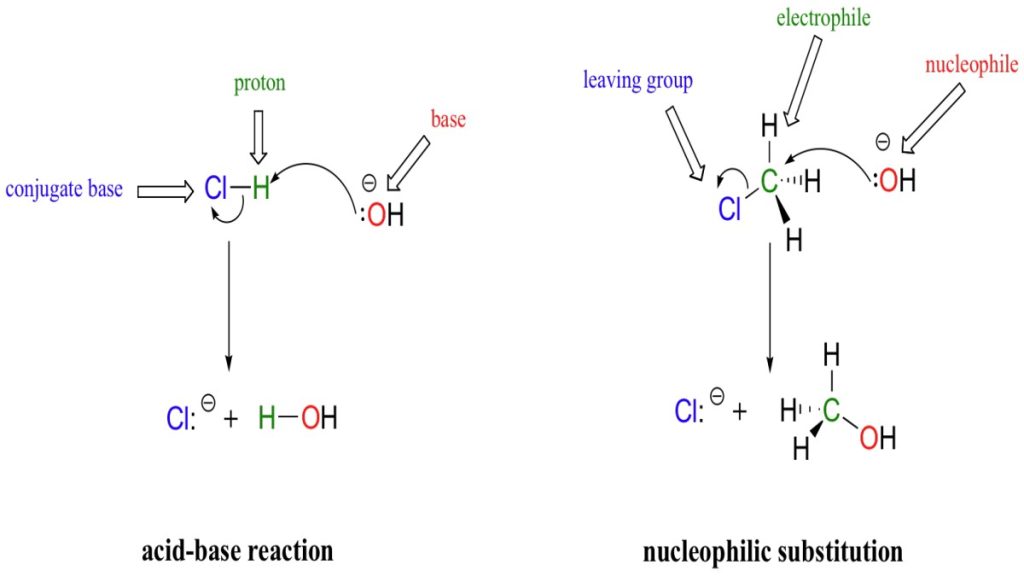
- Nucleophilic substitution is a type of reaction that occurs when an electron rich nucleophile selectively attacks a positively charged (or a partially positive charged) atom in a molecule and replaces a leaving group by bonding with the positively charged species.
Solvolysis is a type of nucleophilic substitution reaction wherein the nucleophile in question is a solvent molecule. A good example of such a nucleophilic solvent is water, and the solvolysis with water is often referred to as hydrolysis.
Ambident Nucleophiles
A Nucleophile which can execute nucleophilic attacks from two or more different places in the molecule (or ion) is called an Ambident Nucleophile. Attacks from these types of nucleophiles can often result in the formation of more than one product.
An example of an ambident nucleophile is the thiocyanate ion which has the chemical formula of SCN– . This ion can execute nucleophilic attacks from either the sulfur atom, or the nitrogen atom. The nucleophilic substitution reactions of alkyl halides involving this ion often result in the formation of a mixture of the following products: alkyl isothiocyanates with the chemical formula R-NCS, and alkyl thiocyanates with the chemical formula R-SCN.
Therefore, an ambident nucleophile can be thought of as an anionic nucleophile in which the negative charge of the ion is delocalized over two different atoms by resonance effects. Commonly, enolate ions exhibit this quality. An illustration of such a resonance structure of an ambident nucleophile is illustrated below.

The electron pair is delocalized over CH2 and O in the example illustrated above.
Types of Nucleophiles
Commonly, the following species form good nucleophiles:
- Halogens – the diatomic form of a halogen does not exhibit nucleophilic qualities. However, the anionic form of these halogens are great nucleophiles. An example of this of this observation is: diatomic iodine (I2) does not act as nucleophile whereas I– is the strongest nucleophile in a polar, protic solvent.
- Carbon – carbon acts as a nucleophile in many organometallic reagents and also in enols. Some examples of compounds wherein carbon acts as a nucleophile include Grignard Reagents, Organolithium Reagents, and n-butyllithium.
- Oxygen – The hydroxide ion is a great example of a nucleophile wherein the electron pair is donated by the oxygen atom. Other examples include alcohols and hydrogen peroxide. It is important to note that no nucleophilic attacks occur during the intermolecular hydrogen bonding that takes place in many compounds containing oxygen and hydrogen.
- Sulfur – due to the large size, the relative ease in its polarization, and the easily accessible lone electron pairs, sulfur has many nucleophilic qualities. Hydrogen sulfide (H2S) is a great example of a nucleophile containing sulfur.
- Nitrogen – Nitrogen is known to form many nucleophiles such as amines, azides, ammonia, and nitrides. Even amides are known to exhibit nucleophilic qualities.
Apart from these specific species listed above, it can be observed that as the ions grow more basic progressing through a row in the periodic table, the nucleophilic reactivity of these ions grows as well.