The respiratory process can be better understood by examining the properties of gases. Gases move freely, but gas particles are constantly hitting the walls of their vessel, thereby producing gas pressure.
Air is a mixture of gases, primarily nitrogen (N2; 78.6 percent), oxygen (O2; 20.9 percent), water vapor (H2O; 0.5 percent), and carbon dioxide (CO2; 0.04 percent). Each gas component of that mixture exerts a pressure. The pressure for an individual gas in the mixture is the partial pressure of that gas. Approximately 21 percent of atmospheric gas is oxygen. Carbon dioxide, however, is found in relatively small amounts, 0.04 percent. The partial pressure for oxygen is much greater than that of carbon dioxide. The partial pressure of any gas can be calculated by:
(39.1) P = (Patm) × (percent content in mixture).
Patm, the atmospheric pressure, is the sum of all of the partial pressures of the atmospheric gases added together,
(39.2) Patm= PN2+ PO2+ PH2O+ PCO2= 760 mm Hg × (percent content in mixture).
The pressure of the atmosphere at sea level is 760 mm Hg. Therefore, the partial pressure of oxygen is:
(39.3) PO2= (760 mm Hg) (0.21) = 160 mm Hg
and for carbon dioxide:
(39.4)PCO2= (760 mm Hg) (0.0004) = 0.3 mm Hg.
At high altitudes, Patm decreases but concentration does not change; the partial pressure decrease is due to the reduction in Patm.
When the air mixture reaches the lung, it has been humidified. The pressure of the water vapor in the lung does not change the pressure of the air, but it must be included in the partial pressure equation. For this calculation, the water pressure (47 mm Hg) is subtracted from the atmospheric pressure:
(39.5)760 mm Hg − 47 mm Hg = 713 mm Hg
and the partial pressure of oxygen is:
(39.6) (760 mm Hg − 47 mm Hg) × 0.21 = 150 mm Hg.
These pressures determine the gas exchange, or the flow of gas, in the system. Oxygen and carbon dioxide will flow according to their pressure gradient from high to low. Therefore, understanding the partial pressure of each gas will aid in understanding how gases move in the respiratory system.
Gas Exchange across the Alveoli
In the body, oxygen is used by cells of the body’s tissues and carbon dioxide is produced as a waste product. The ratio of carbon dioxide production to oxygen consumption is the respiratory quotient (RQ). RQ varies between 0.7 and 1.0. If just glucose were used to fuel the body, the RQ would equal one. One mole of carbon dioxide would be produced for every mole of oxygen consumed. Glucose, however, is not the only fuel for the body. Protein and fat are also used as fuels for the body. Because of this, less carbon dioxide is produced than oxygen is consumed and the RQ is, on average, about 0.7 for fat and about 0.8 for protein.
The RQ is used to calculate the partial pressure of oxygen in the alveolar spaces within the lung, the alveolar PO2 Above, the partial pressure of oxygen in the lungs was calculated to be 150 mm Hg. However, lungs never fully deflate with an exhalation; therefore, the inspired air mixes with this residual air and lowers the partial pressure of oxygen within the alveoli. This means that there is a lower concentration of oxygen in the lungs than is found in the air outside the body. Knowing the RQ, the partial pressure of oxygen in the alveoli can be calculated:
(39.7) 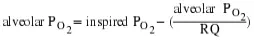
With an RQ of 0.8 and a PCO2 in the alveoli of 40 mm Hg, the alveolar PO2
is equal to:
(39.8) 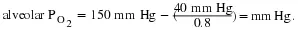
Notice that this pressure is less than the external air. Therefore, the oxygen will flow from the inspired air in the lung (PO2 = 150 mm Hg) into the bloodstream (PO2 = 100 mm Hg)
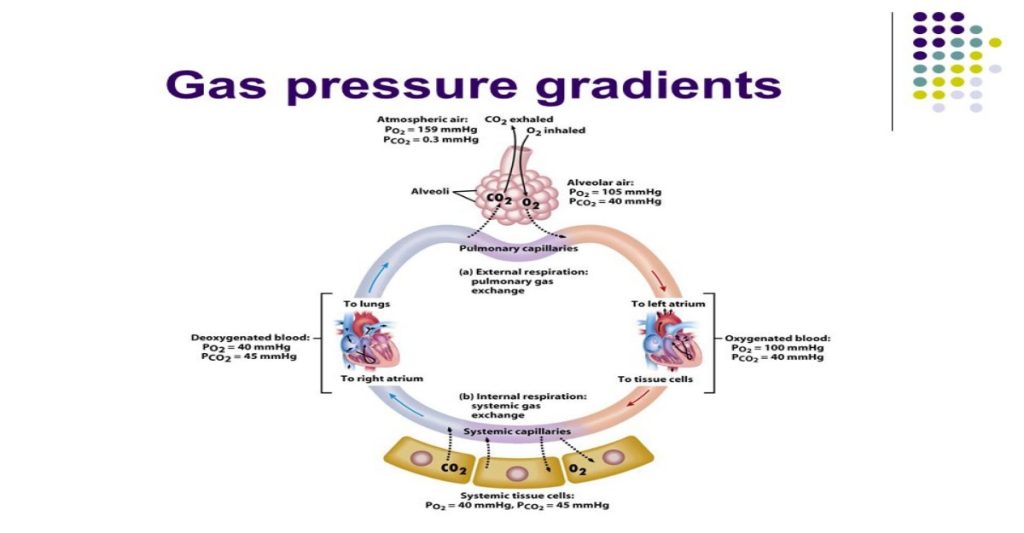
(Figure 20.13).
In the lungs, oxygen diffuses out of the alveoli and into the capillaries surrounding the alveoli. Oxygen (about 98 percent) binds reversibly to the respiratory pigment hemoglobin found in red blood cells (RBCs). RBCs carry oxygen to the tissues where oxygen dissociates from the hemoglobin and diffuses into the cells of the tissues. More specifically, alveolar PO2 is higher in the alveoli (PALVO2 = 100 mm Hg) than blood PO2 (40 mm Hg) in the capillaries. Because this pressure gradient exists, oxygen diffuses down its pressure gradient, moving out of the alveoli and entering the blood of the capillaries where O2 binds to hemoglobin. At the same time, alveolar PCO2 is lower PALVO2 = 40 mm Hg than blood PCO2 = (45 mm Hg). CO2 diffuses down its pressure gradient, moving out of the capillaries and entering the alveoli.
Oxygen and carbon dioxide move independently of each other; they diffuse down their own pressure gradients. As blood leaves the lungs through the pulmonary veins, the venous PO2= 100 mm Hg, whereas the venous PCO2 = 40 mm Hg. As blood enters the systemic capillaries, the blood will lose oxygen and gain carbon dioxide because of the pressure difference of the tissues and blood. In systemic capillaries, PO2= 100 mm Hg, but in the tissue cells, PO2= 40 mm Hg. This pressure gradient drives the diffusion of oxygen out of the capillaries and into the tissue cells. At the same time, blood PCO2= 40 mm Hg and systemic tissue PCO2= 45 mm Hg. The pressure gradient drives CO2 out of tissue cells and into the capillaries. The blood returning to the lungs through the pulmonary arteries has a venous PO2= 40 mm Hg and a PCO2= 45 mm Hg. The blood enters the lung capillaries where the process of exchanging gases between the capillaries and alveoli begins again (Figure 20.13).
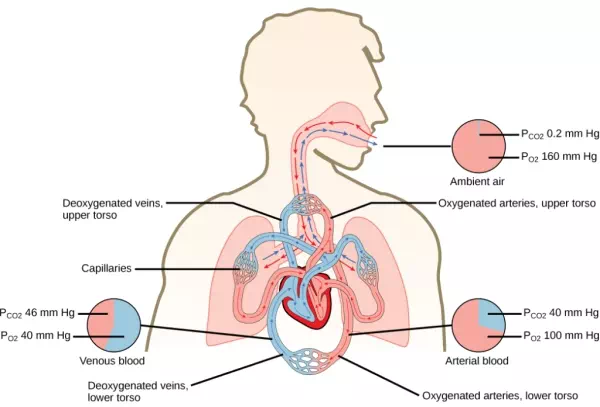
Figure 20.13. The partial pressures of oxygen and carbon dioxide change as blood moves through the body.
Which of the following statements is false?
1. In the tissues, PO2 drops as blood passes from the arteries to the veins, while PCO2 increases.
2. Blood travels from the lungs to the heart to body tissues, then back to the heart, then the lungs.
3. Blood travels from the lungs to the heart to body tissues, then back to the lungs, then the heart.
4. PO2 is higher in air than in the lungs.
In short, the change in partial pressure from the alveoli to the capillaries drives the oxygen into the tissues and the carbon dioxide into the blood from the tissues. The blood is then transported to the lungs where differences in pressure in the alveoli result in the movement of carbon dioxide out of the blood into the lungs, and oxygen into the blood.
Summary
The lungs can hold a large volume of air, but they are not usually filled to maximal capacity. Lung volume measurements include tidal volume, expiratory reserve volume, inspiratory reserve volume, and residual volume. The sum of these equals the total lung capacity. Gas movement into or out of the lungs is dependent on the pressure of the gas. Air is a mixture of gases; therefore, the partial pressure of each gas can be calculated to determine how the gas will flow in the lung. The difference between the partial pressure of the gas in the air drives oxygen into the tissues and carbon dioxide out of the body.