Specimen
- Dacron swabs from the nose, throat, or other suspected lesions must be obtained before antimicrobial drugs are administered.
- Swabs should be collected from beneath any visible membrane.
- The swab should then be placed in semisolid transport media such as Amies.
Microscopy
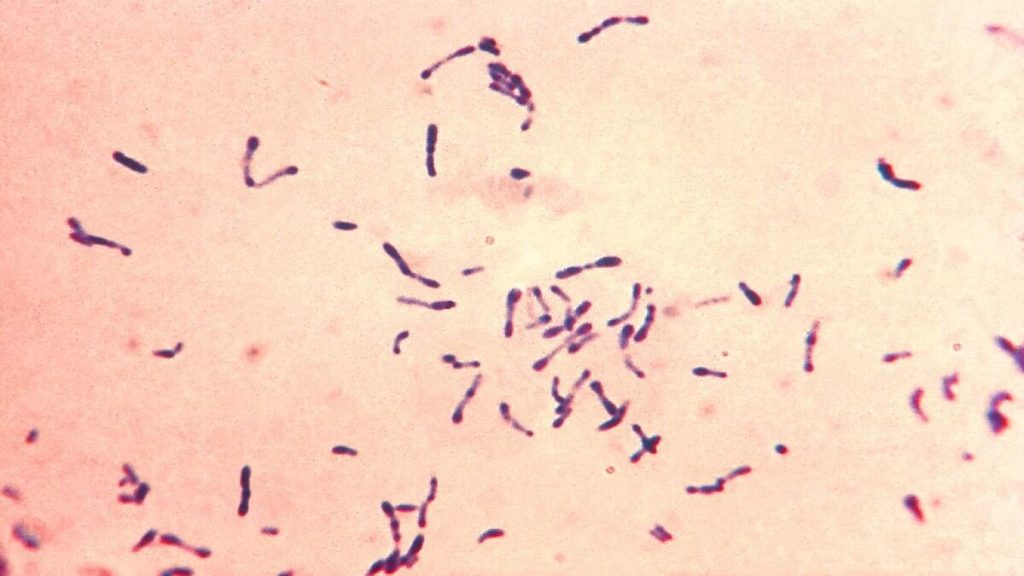
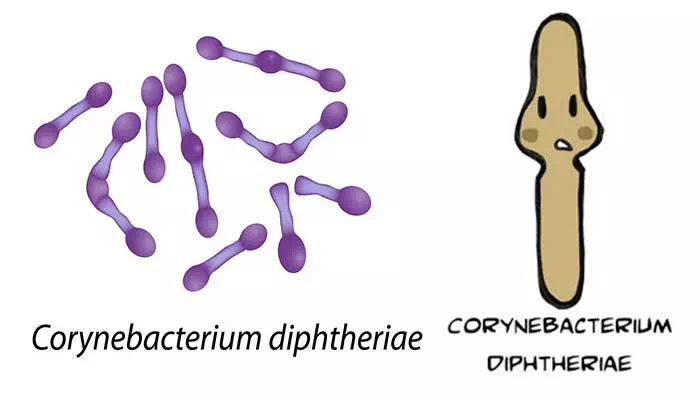
- Smears stained with alkaline methylene blue or Gram stain show beaded rods in typical arrangement.
- Cells often contain metachromatic granules (polymetaphosphate), which stain bluish-purple with methylene blue.
- In Gram staining, purple coloured beaded rods, angular and palisade arrangements that creates a ‘Chinese character’ effect is observed.
Culture
- Specimens should be inoculated to a blood agar plate and a selective medium such as a tellurite plate (eg: cystine-tellurite blood agar [CTBA] or modified Tinsdale’s medium) and incubated at 37°C in 5% CO2.
- Non β hemolytic region on blood agar.
- Tellurite inhibits the growth of most upper respiratory tract bacteria and gram-negative rods and is reduced by C. diphtheriae, producing characteristic gray to black color on agar.
- Degradation of cysteine by C. diphtheriae cysteinase activity produces a brown halo around the colonies.
- Tinsdale medium is the best medium for recovering C. diphtheriae in clinical specimens, but it has a short shelf life and requires addition of horse serum.
Biochemical testing
Catalase – positive
Oxidase – negative
Cystinase production – positive
Pyrazinamidase activity – positive
Carbohydrate fermentation
Glucose – positive
Maltose – positive
Sucrose – negative
Trehalose – negative
Urease – negative
Nitrate reduction – positive
Gelatin liquefaction – negative
Toxigenicity Testing
- All isolates of C. diphtheriae should be tested for the production of exotoxin.
- The gold standard for detection of diphtheria toxin is an in-vitro immunodiffusion assay by Elek-Ouchterlony immunodiffusion test (Elek test).
- An alternative method is detection of the exotoxin gene using a polymerase chain reaction (PCR) based nucleic acid amplification method.
- This test can detect the tox gene in clinical isolates and directly in clinical specimens (e.g., swabs from the diphtheritic membrane or biopsy material).
- A positive culture result confirms a positive PCR assay.
- A negative culture result after antibiotic therapy along with a positive PCR assay result suggests that the patient probably has diphtheria.
- Although this test is rapid and specific, strains in which the tox gene is not expressed can give a positive signal.
- Enzyme-linked immunosorbent assays can be used to detect diphtheria toxin from clinical C diphtheria isolates.
- An immunochromatographic strip assay allows detection of diphtheria toxin in a matter of hours.
- However ELISA and immunochromatographic assay are not widely used.
Antibody detection
- Measurement of antibodies to diphtheria toxin in serum collected before administration of antitoxin may support the diagnosis when cultures are negative.
Treatment of Corynebacterium diphtheriae
- The early administration of specific antitoxin against the toxin formed by the organisms at their site of entry and multiplication is done promptly.
- The antitoxin should be given intravenously on the day the clinical diagnosis of diphtheria is made and need not be repeated.
- Intramuscular injection may be used in mild cases.
- Diphtheria antitoxin will only neutralize circulating toxin that is not bound to tissue.
- Antimicrobial drugs (penicillin, macrolides) inhibit the growth of diphtheria bacilli.
- Although these drugs have virtually no effect on the disease process, they arrest toxin production and assist public health efforts.
- Erythromycin may be preferred to penicillin for elimination of the bacilli from the throat, particularly in treatment of persistent carriers.
- Some strains are tolerant to the bactericidal action of penicillins, and treatment of complicated infections should contain an association with an aminoglycoside.
- Patients should be placed in strict isolation, nursed by staff whose immunization history is documented and have daily platelet counts and electrocardiography.
Prevention and control of Corynebacterium diphtheriae
- Active immunization in childhood with diphtheria toxoid yields antitoxin levels that are generally adequate until adulthood.
- Diphtheria toxoids are commonly combined with tetanus toxoid (Td) and with a cellular pertussis vaccine (DaPT) as a single injection to be used in initial immunization of children (three doses in the first year of life, 15–18 months of age and 4–6 years of age).
- Young adults should be given boosters of toxoid because toxigenic diphtheria bacilli are not sufficiently prevalent in the population to provide the stimulus of subclinical infection with stimulation of resistance.
- Levels of antitoxin decline with time, and many older persons have insufficient amounts of circulating antitoxin to protect them against diphtheria.
- The principal aims of prevention are to limit the distribution of toxigenic diphtheria bacilli in the population and to maintain as high a level of active immunization as possible.
- Bed rest, isolation to prevent secondary spread, and maintenance of an open airway in patients with respiratory diphtheria are all important.