
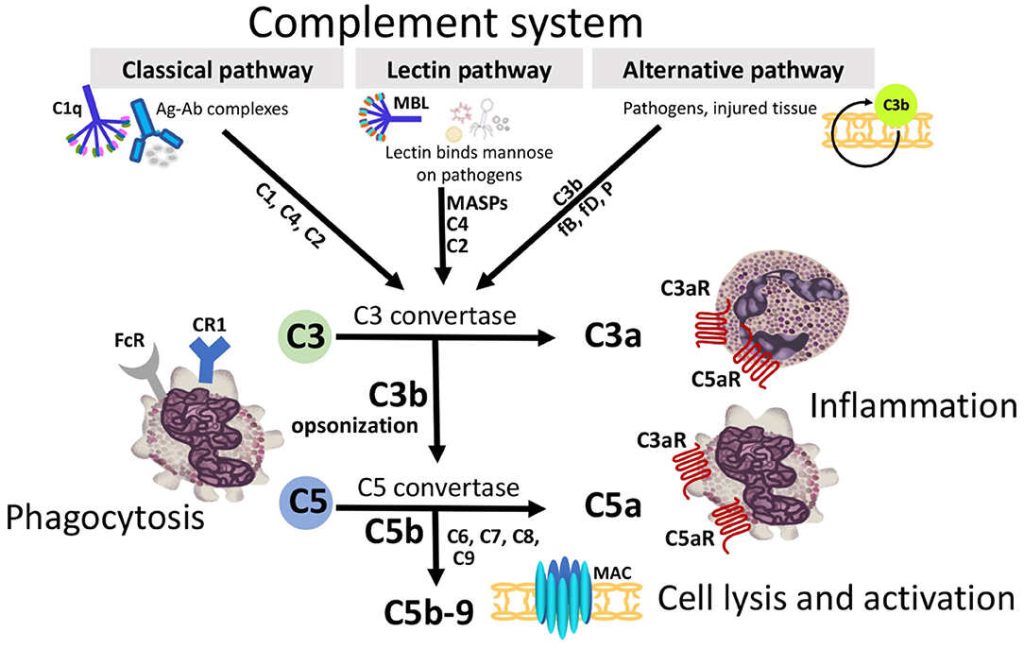
The complement system is a part of the immune system, consists of a series of proteins that interact with one another in a highly regulated manner, in order to eliminate pathogens. It helps antibodies and phagocytic cells to clear pathogens and damaged cells; promote inflammation and attack pathogen’s plasma membrane. Proteins that take part in the complement system are called complements that collectively work as a biological cascade; the sequence of reactions, each being the catalyst for the next.
Jules Bordet (1895) identified complements as heat-sensitive components in the blood, bearing non-specific antimicrobial activity.
Complements are soluble proteins and glycoproteins mostly produced by hepatocytes. More than 20 types of complements are present in serum, found circulating normally in human body in inactive forms (called as zymogens or proenzymes). Complement activation is triggered by an antibody when it is bound to the antigen. It can also be triggered by some components of innate immunity. Thus the complement system works in both innate and acquired immunity.
Complements are activated only during inflammatory reactions. During the inflammation, more amount of complements reaches to the interstitial area of the infected tissue through dilated blood vessels, which are then activated by proteolytic cleavage; this exposes the active site of the complements.
Complements are mainly denoted by the capital letter C with numbers; like, C1, C2, C3, and so on. Some have only alphabet, like, B, D. Some are simply represented by names, like, homologous restriction factor.
C1 has three sub-units; C1q, C1r and C1s. C2-C5 have two components, a and b. Larger subunits are denoted by b whereas the smaller are denoted by a (except C2a, which is larger than C2b).
Complement Activation and cell lysis
The complement activation occurs via three pathways; which are:
1. Classical pathway
2. Alternative pathway
3. Lectin pathway (or mannose binding lectin pathway)
C5 convertase, generated by the alternative, classical, or lectin pathway, initiates the activation of late components of the complement system to form membrane attack complex (MAC) and ultimately kills the pathogen.

This occurs through three pathways; Classical pathway, activated by antigen-antibody reaction, Alternative pathway, activated on microbial cell surfaces, and Mannose binding Lectin pathway, activated by a plasma lectin that binds to mannose residues on microbes.
1. Classical Pathway
The classical pathway begins with the formation of antigen-antibody complex (immune complex). When an antigen enters the body, the antibody (IgM/IgG) binds to it. This induces conformational changes in the Fc portion of the antibody which exposes a binding site for C1 protein. Hence, the antibody activates the complement system only when bound to an antigen.
C1 is a large, multimeric, protein complex composed of one molecule of C1q and two molecules each of C1r and C1s subunits. C1q binds to the antigen bound antibody (Fc portion). C1r and C1s are proteases which help to cleave C4 and C2.
The immune complex bound to C1 calls another protein C4 which is cleaved into C4a and C4b. C4a goes away whereas activated C4b attaches to the target surface near C1q. Now, C4b attracts C2 which is also cleaved into C2a and C2b. C2a binds C4b forming the C4b2a complex whereas C2b goes away. The active C4bC2a activates C3. The C4b2a complex is also known as C3 convertase as this converts C3 into an active form by separating C3a and C3b. One molecule of C4b2a can cleave a large number of C3 molecules. C3b binds to the microbial surface or to the convertase itself.
C3b when binds to C3 convertase forms C4bC2aC3b (C5 convertase) which activates C5.
C5 convertase cleaves C5 into C5a and C5b. C5a diffuses away but C5b is stabilized by binding C6. Then C5bC6 binds to C7. C5bC6C7 complex is then inserted into the phospholipid bilayer of the cell membrane which further binds C8. These all (C5b678) activate C9 to form a macromolecular structure called the membrane attack complex (MAC). This makes hole in the bacterium, as a result, the intracellular contents leak out and unwanted substances get in. Thus, the cell cannot maintain its osmotic stability and the lysis occurs by an influx of water and loss of electrolytes.
This is more effective in Gram negative bacteria than in Gram positive bacteria because MAC formation is easy in the outer membrane in Gram negatives whereas it is difficult in the rigid thick layer of peptidoglycan in Gram positives.
Some of the C3b molecules do not associate with C4b2a; instead these molecules coat immune complexes or microbial cell surfaces and work as opsonins. This process is called opsonization in which opsonin molecule binds one side to the particulate matter i.e. in bacteria, tumor cell, RBC and on the other side they bind to the receptor of phagocytic cell(like, neutrophils and macrophages) which enhance the process of phagocytosis.
Smaller complement subunits diffuse from the site and can initiate localized inflammatory responses by binding to specific receptors.
2. Alternative Pathway
Unlike classical pathway, alternative pathway, does not require Ag-Ab complex for the initiation of complement pathway. It is initiated by cell surface constituents that are foreign to the host. These surface molecules may be lipopolysaccharide etc.
When a bacterium enters the host body, as a result of inflammation, complements reach towards the site, where C3 molecules directly touch antigen and become active. In this pathway, serum C3 containing an unstable thioester bond undergoes slow spontaneous hydrolysis to yield C3a and C3b. C3b binds the surface of foreign cell and then binds to another serum protein called factor B. Now the factor B exposes the site which serves as the substrate for enzymatically active serum protein D. Then factor D cleaves B into Ba and Bb forming C3 convertase (C3bBb). C3 convertase then forms C5 convertase which ultimately forms a MAC as in classical pathway.

3. Mannose binding Lectin (MBL) Pathway
Some bacteria can activate complement system without having antibody and endotoxin. This occurs through MBL pathway which is activated when circulating lectin (MBL) binds to mannose residues on glycoproteins or carbohydrates on the surface of microorganisms. Microorganisms inducing MBL pathway are bacteria, such as Salmonella, Listeria, and Neisseria strains, some fungi and some viruses including HIV-1. MBL is an acute phase protein and its concentration increases during inflammation. The lectin recognizes and binds the carbohydrate of the target cell which then activates complements.
MBL pathway resembles classical pathway as it proceeds through the action of C4 and C2 to produce activated proteins of the complement system. MBL works same as C1q which it resembles in structure.
After the MBL binds to carbohydrate residues on the surface of a cell or pathogen, two components, MASP-1 and MASP-2 bind to MBL. MASP stands for MBL-associated serine proteases. Two proteases form a tetrameric complex similar to the one formed by C1r and C1s and cleaves C4 and C2 forming C3 convertase. The process now continues to form of C5 convertase and the MAC as in classical pathway.
Functions of Complements

Function of Complement Pathway
Some major functions of complements are:
1. Opsonization and phagocytosis
C3b, bound to immune complex or coated on the surface of pathogen, activate phagocytic cells. These proteins bind to specific receptors on the phagocytic cells to get engulfed.
2. Cell lysis
Membrane attack complex formed by C5b6789 components ruptures the microbial cell surface which kills the cell.
3. Chemotaxis
Complement fragments attract neutrophils and macrophages to the area where the antigen is present. These cell surfaces have receptors for complements, like C5a, C3a, thus, run towards the site of inflammation, i.e. chemotaxis.
4. Activation of mast cells and basophils and enhancement of inflammation
The proteolytic complement fragments, C5a, C4a, and C3a induce acute inflammation by activating mast cells and neutrophils. All three peptides bind to mast cells and induce degranulation, with the release of vasoactive mediators such as histamine. These peptides are also called anaphylatoxins because the mast cell reactions they trigger are characteristic of anaphylaxis. Binding to specific complement receptors on cells of the immune system, they trigger specific cell functions, inflammation, and secretion of immunoregulatory molecules.
5. Production of antibodies
B cells have receptor for C3b. When C3b binds to B-cell, it secretes more antibodies. Thus C3b is also an antibody producing amplifiers which converts it into an effective defense mechanism to destroy invading microorganism.
6. Immune clearance
The complement system removes immune complexes from the circulation and deposits them in the spleen and liver. Thus it acts as anti-inflammatory function. Complement proteins promote the solubilization of these complexes and their clearance by phagocytes.
Complement regulation
The complement system has the potential to be extremely damaging to host tissues; hence regulatory mechanisms are required to restrict the complement pathway. Various plasma and cell membrane proteins regulate complement activation by inhibiting different steps in the cascade.
The membrane of most mammalian cells has a high level of sialic acid, which contributes to the inactivation of complements.
Complement related Diseases
Diseases associated with complements can be due to the deficiencies in any of the protein components or in regulatory components.
Some examples of complement protein deficiencies are:
Deficiency of C2 and C4 can cause systemic lupus erythematosus; deficiency of C3 and factor D can cause pyogenic bacterial infection; and deficiency of C5-C9 (or MAC deficiency) may lead to the Neisserial infections like, gonorrhea and meningitis.
Deficiencies of regulatory proteins lead to too much activation of complements in wrong time and place which leads to unwanted inflammation and cell lysis. Pyogenic bacterial infection and glomerulonephritis are the results of such deficiencies.
Mutations in the complement regulators factors may lead to atypical hemolytic uremic syndrome, age-related macular degeneration, hereditary angioedema, etc.
Complement system can also be stimulated by abnormal stimuli, like persistent microbes, antibody against self antigens or immune complexes deposited in tissues. Even when the system is properly regulated and activated, it can cause significant tissue damage.
Comments are closed