
Capsule stain is a type of differential stain which uses acidic and basic dyes to stain background & bacterial cells respectively so that presence of capsule is easily visualized. Capsule is synthesized in the cytoplasm and secreted to the outside of the cell where it surrounds the bacterium. Most of the capsulated bacteria have a capsule made up of polysaccharide layer but some bacteria have capsule made up of polypeptide, or glycoprotein.
Principle of Capsule Stain
Bacterial capsules are non-ionic, so neither acidic nor basic stains will adhere to their surfaces. Therefore, the best way to visualize them is to stain the background using an acidic stain (e.g., Nigrosine, congo red) and to stain the cell itself using a basic stain (e.g.,crystal violet, safranin, basic fuchsin and methylene blue).

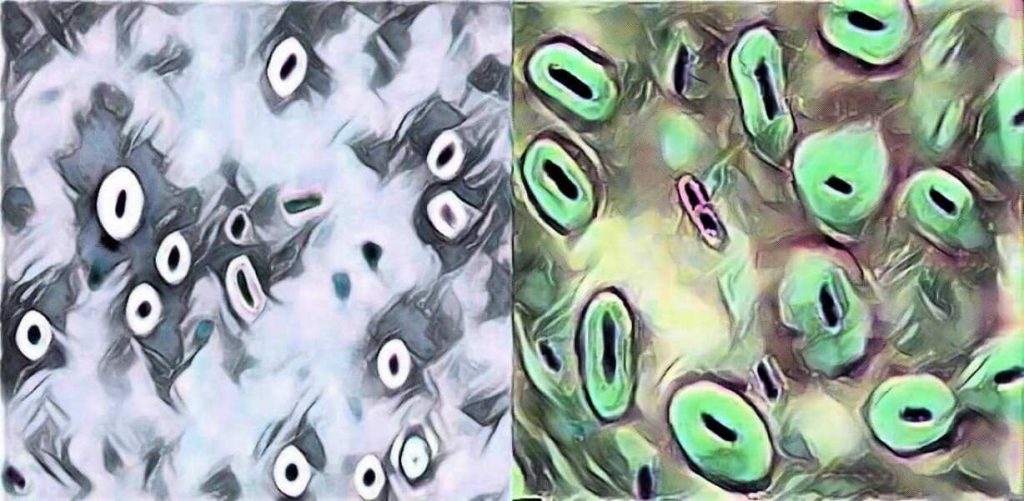
Image 1: Capsule Staining
Various types of methods are available for the demonstration of the presence of capsule. The results (stain of the cells, background and capsule) depend on the type of the method used. Two commonly used methods are discussed here:
A. India ink method
In this method two dyes, crystal violet and india ink are used. The capsule is seen as a clear halo around the microorganism against the black background. This method is used for demonstrating Cryptococcus.
§ The background will be dark (color of india ink).
§ The bacterial cells will be stained purple (bacterial cells takes crystal violet-basic dyes as they are negatively charged).
§ The capsule (if present) will appear clear against the dark background (capsule does not take any stain).
B. Anthony’s stain method
In this type of capsule staining procedure, the primary stain is crystal violet, and all parts of the cell take up the purple crystal violet stain. There is no mordant in the capsule staining procedure. A 20% copper sulfate solution serves a dual role as both the decolorizing agent and counter stain. It decolorizes the capsule by washing out the crystal violet, but will not decolorize the cell. As the copper sulfate decolorizes the capsule, it also counter stains the capsule. Thus, the capsule appears as a faint blue halo around a purple cell.
Materials and reagents required
§ Test bacteria: 36-48 hour culture of capsulated bacteria e.g. Klebsiella pneumoniae growing on a slant of EMB Agar or culture of other capsulated bacteria and non-capsulated bacteria [Note:Growing Klebsiella pneumoniae in milk based media (e.g. Skim milk) increase its capsule size, making it easier to visualize.]
§ Stain solutions: Depending on the types of method used (Crystal violet, India ink, Nigrosin, Copper Sulfate, Basic carbol fuschin solution, Methylene blue solution etc).
§ Microscopic slides
§ Inoculating loop
§ Microscope with 100x objective lens (oil immersion)
§ Immersion oil
§ Gas burner
§ Tissue paper
Capsule Stain procedure
A. India Ink Method
1. Place a single drop of India ink on a clean microscope slide, adjacent to the frosted edge.
2. Using a flamed loop and sterile technique, remove some Klebsiella pneumoniae from culture tube or plate and mix it into the drop of India ink. Be sure there are no large clumps of organism, but try to avoid spreading the drop.
3. Place the end of another clean microscope slide at an angle to the end of the slide containing the organism. Spread out the drop out into a film. This is done by contacting the drop of India ink with the clean microscope slide and using the capillary action of the dye/ slide to spread the India ink across the smear.
4. Allow the film to air dry (will take 5-7 minutes). DO NOT heat or blot dry! Heat will melt the capsule!
5. Saturate the slide with crystal violet for 1 minute and rinse slightly & very gently with water. Be cautious water may remove the capsule from the cell.

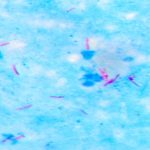
Image 2: Capsule staining by India ink method (at 1000x magnification)
6. Let the slide air dry for a few minutes. DO NOT blot the slide! Blotting will remove the bacteria from the slide and/or distort the capsule.
7. Observe the slide under oil immersion.
Results: Look for purple cells surrounded by a clear halo on a dark background. The halo is the capsule. You may need to decrease the amount of light in order to make the capsule easier to see.
B. Anthony’s stain method
1. Place a single drop of crystal violet on a clean microscope slide, adjacent to the frosted edge.
2. Using a flamed loop and sterile technique, add three loopful of test bacterium (any capsulated bacteria such as Klebsiella pneumoniae, Streptococcus pneumoniae) from broth culture. If you are adding bacteria from a culture plate make sure that there are no large clumps of organism, but try to avoid spreading the drop.
3. Place the end of another clean microscope slide at an angle to the end of the slide containing the organism. Spread out the drop out into a film. This is done by contacting the drop of crystal violet with the clean microscope slide and using the capillary action of the dye/ slide to spread the crystal violet across the smear.
4. Allow the film to air dry (will take 5-7 minutes). DO NOT heat or blot dry! Heat will melt the capsule!
5. Tilt the slide and rinse with 20% copper sulfate solution. DO NOT RINSE WITH WATER! Water will remove the capsule from the cell.
6. Let the slide air dry for a few minutes. DO NOT blot the slide! Blotting will remove the bacteria from the slide and/or distort the capsule.
7. Observe the slide under oil immersion.
Comments are closed