INTENDED USE
Hardy Diagnostics Flagella Stain is recommended for use in detecting the presence and arrangement of flagella on the bacterial cell.
SUMMARY
Bacterial flagella, due to their narrow diameter, cannot be seen with a light microscope. The Flagella Stain provides a method for viewing bacterial flagella by employing crystal violet in an alcoholic solution as the primary stain. During the staining procedure, the alcoholic solution evaporates and leaves a precipitate around the flagella, increasing its apparent size to the human eye. The Flagella Stain also contains tannic acid and aluminum potassium phosphate as mordants, and phenol as an antifungal agent.
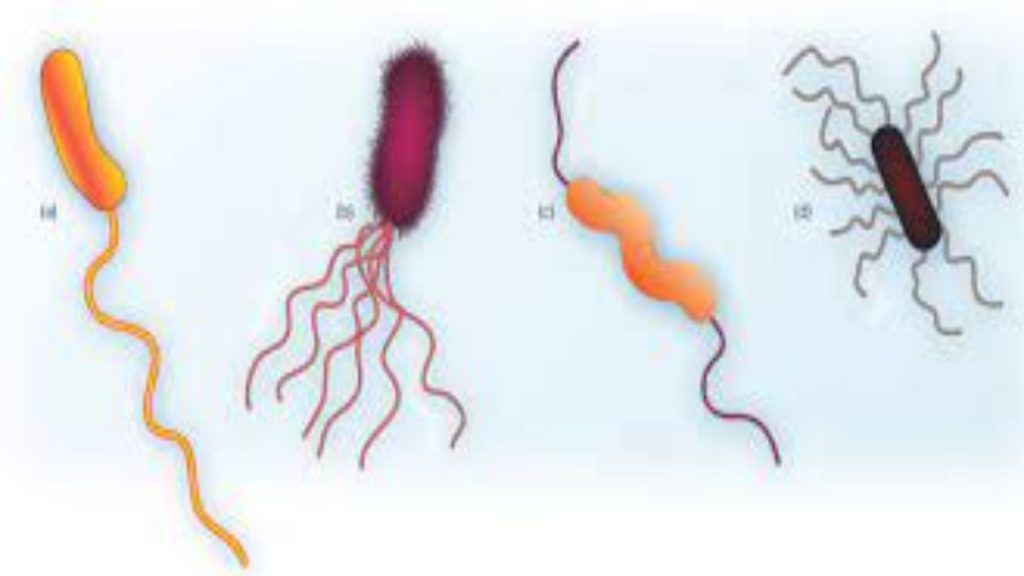
Hardy Diagnostics Flagella Stain is a simple, rapid, qualitative method for detecting bacterial flagella and their shape, length, curvature, arrangement and number on the cell. (2,5) This method was developed by Ryu in 1937, and also later described by Kodaka, et al. in 1982. This test has been found especially useful in providing taxonomic and identifying information about motile bacteria, and more recently, anaerobic bacteria.
REAGENT FORMULA
Ingredients per liter of deionized water:*
| Aluminum Potassium Sulfate | 57.0gm |
| Phenol | 25.0gm |
| Tannic Acid | 20.0gm |
| Crystal Violet, Certified | 6.0gm |
* Adjusted and/or supplemented as required to meet performance criteria.
STORAGE AND SHELF LIFE
Storage: Upon receipt store at 2-30ºC., in the dark. Product should not be used if there are any signs of deterioration or if the expiration date has passed. Do not expose to excessive heat or moisture. Product is light sensitive; protect from light. The expiration dating on the product label applies to the product in its intact packaging when stored as directed. The product may be used and tested up to the expiration date on the product label and incubated for the recommended quality control incubation times.
PRECAUTIONS
This product may contain components of animal origin. Certified knowledge of the origin and/or sanitary state of the animals does not guarantee the absence of transmissible pathogenic agents. Therefore, it is recommended that these products be treated as potentially infectious, and handle observing the usual universal blood precautions. Do not ingest, inhale, or allow to come into contact with skin. This product is for in vitro diagnostic use only. It is to be used only by adequately trained and qualified laboratory personnel. Observe approved biohazard precautions and aseptic techniques. All laboratory specimens should be considered infectious and handled according to “standard precautions.”
For additional information regarding specific precautions for the prevention of the transmission of all infectious agents from laboratory instruments and materials, and for recommendations for the management of exposure to infectious disease, refer to CLSI document M-29: Protection of Laboratory Workers from Occupationally Acquired Infections: Approved Guideline.
Warning! Contains Phenol! Harmful if swallowed, inhaled, or absorbed through skin. Causes irritation. Avoid contact with eyes, skin mucous membranes, and clothing. If contact occurs, flush immediately with large amounts of water for at least 15 minutes, remove contaminated clothing, and call a physician. Avoid breathing gas, vapor, dust or mist. Wash clothing before reuse. Keep container tightly closed at all times.
Warning! Staining solutions are hazardous in nature. Wear appropriate safety apparel when working with staining solutions. It is recommended that staining procedures be performed under a hood and with adequate ventilation.
PROCEDURE
Specimen Collection: This product is not intended for primary isolation of patient specimens. It should be used only with cultures of isolated organism. This product used with other biochemical tests to identify cultures of isolated organism. Consult listed references for information on specimen collection.
Organism Preparation:
For best results it is recommended that an 18-48 hour old, well isolated colony taken from a Blood Agar plate be used for the Flagella Stain. Streak the organism to be examined for isolation on a Blood Agar plate (Cat. no. A10). Incubate in an appropriate environment at 35ºC. Some non-fermentative organisms grow better at 25ºC., and are more likely to be flagellated at this growth temperature.
Smear Preparation:
1. With a wax pencil, draw a border around the clear portion of a microscope slide.
2. Approximately one centimeter from the frosted edge, place a drop of deionized (or distilled) water.
3. With a sterile inoculating loop, gently touch a colony of the culture to be examined and then lightly touch the drop of water without touching the slide. Do not mix.
4. Tilt the slide so the drop will flow to the opposite end of the slide.
5. Allow the slide to air dry, at room temperature. Do not heat fix.
Staining Procedure:
1. Flood the slide with Flagella Stain. Let the slide sit for approximately four minutes. However, staining time may need to be adjusted to between two to eight minutes, depending on the age of the stain, air currents and room temperature, and staining solution depth over the smear. (5,6)
2. Carefully rinse stain by leaving slide on staining rack and allowing water to flow over the surface of the slide. Do not tilt slide while rinsing.
3. Once the stain is washed off, gently tilt the slide to allow excess water to run off.
4. Air dry slide at room temperature.
5. Examine the slide microscopically using the oil immersion objective. Begin the examination at thinner areas of the smear and work towards the center. Look for fields with several isolated cells rather than clumps of bacteria.
INTERPRETATION OF RESULTS
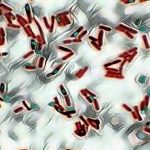
When examined under oil immersion, bacteria and their flagella should stain purple. Consult appropriate references for descriptions, drawings or pictures for specific information. (2,4,5,7)
LIMITATIONS
The presence of fermentable substances in the growth medium, age of the culture, and the temperature of incubation have all been shown to affect the degree of flagellation.(5) For optimal results, use cultures of log phase of organism from Blood Agar plates (Cat. no. A10). Care is needed in transferring organisms from the culture to the water droplet on the slide, as flagella are easily dislodged from the bacterial cells. The length of staining time may affect staining quality. A short staining time may result in poorly stained flagella. If this occurs, increase staining time. Prolonged staining may result in noticeable precipitation of the crystal violet on the slide. In this case, decrease the staining time.
MATERIALS REQUIRED BUT NOT PROVIDED
Standard microbiological supplies and equipment such as loops, glass slides, wax pencils, immersion oil, culture media, pipets, microscopes, incinerators, and incubators, etc., as well as serological and biochemical reagents, are not provided.
QUALITY CONTROL
Hardy Diagnostics tests each lot of commercially manufactured media using appropriate quality control microorganisms and quality specifications as outlined on the Certificates of Analysis (CofA). The following organisms are routinely used for testing at Hardy Diagnostics:
| Test Organisms | Results |
| Klebsiella pneumoniae ATCC® 13883 | No flagella seen |
| Proteus vulgaris ATCC® 8427 | Peritrichous flagella seen |
| Pseudomonas aeruginosa ATCC® 27853 | Monotrichous flagella seen |
USER QUALITY CONTROL
End users of commercially prepared culture media should perform QC testing in accordance with applicable government regulatory agencies, and in compliance with accreditation requirements. Hardy Diagnostics recommends end users check for signs of contamination and deterioration and, if dictated by laboratory quality control procedures or regulation, perform quality control testing to demonstrate growth or a positive reaction and to demonstrate inhibition or a negative reaction, if applicable. Hardy Diagnostics quality control testing is documented on the certificates of analysis (CofA) available from Hardy Diagnostics Certificates of Analysis website. In addition, refer to the following document “Finished Product Quality Control Procedures,” for more information on QC or see reference(s) for more specific information. It is recommended that each new lot of stain be tested with known positive and negative controls and retested each week of use thereafter. The microscope be calibrated (within the last 12 months), and the objectives and oculars used for the calibration procedure should be in place on the microscope when objects are measured. It is recommended that positive controls be run in parallel with patient specimens and that results from this staining procedure be reported only if positive control smears are acceptable.
Comments are closed