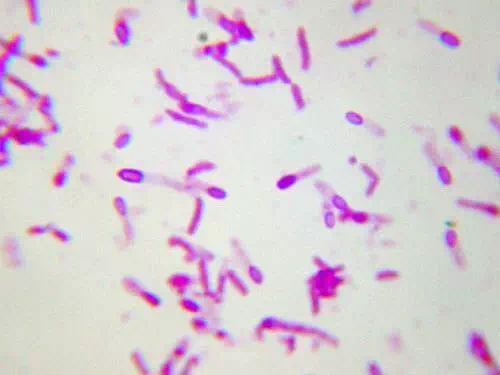
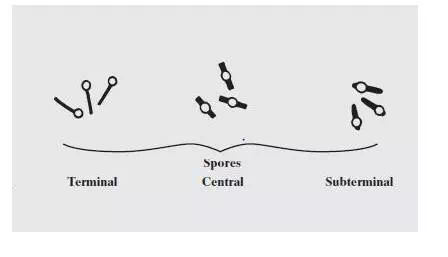
When vegetative cells of certain bacteria such as Bacillus spp and Clostridium spp are subjected to environmental stresses such as nutrient deprivation, they produce metabolically inactive or dormant form-endospore. Formation of endospore circumvent the problems associated with environmental stress and helps them to survive. During unfavorable conditions (especially when carbon and nitrogen become unavailable) endospores can form within different areas of the vegetative cell. They can be central, subterminal, or terminal. Central endospores are located within the middle of the vegetative cell. Terminal endospores are located at the end of the vegetative cell. Sub-terminal endospores are located between the middle and the end of the cell.
Find more information about Endospore Structure and Importance Here
Most endospore forming bacteria are found in soil or aquatic environments. However, some species of Bacillus and Clostridium have medical significance. Clostridium perfringens, C. botulinum (a potential agent of bioterrorism) and C. tetani are the causative agents of gas gangrene, botulism and tetanus, respectively. Bacillus anthracis and Bacillus cereus are the causative agents of anthrax and a self limiting food poisoning, respectively.
Principle of Spore Staining:
A differential staining technique (the Schaeffer-Fulton method) is used to distinguish between the vegetative cells and the endospores. A primary stain (malachite green) is used to stain the endospores. Because endospores resist staining, the malachite green will be forced into (i.e, malachite green permeate the spore wall) the endospores by heating. In this technique heating acts as a mordant. There is no need of using any decolorizer in this spore staining as the primary dye malachite green bind relatively weakly to the cell wall and spore wall .In fact If washed well with water the dye come right out of cell wall however not from spore wall once the dye is locked in. Water is used to decolorize the vegetative cells.
( Note: In Gram Staining and AFB Staining we use Alcohol or Acid Acohol or Acid as a decolorizer but in spore staining water is sufficient ( to be used as decolorizer) because:
· malachite green dye is water-soluble and does not adhere well to the cell wall
· vegetative cells have been disrupted by heat,
because of these reasons, the malachite green rinses easily from the vegetative cells. )
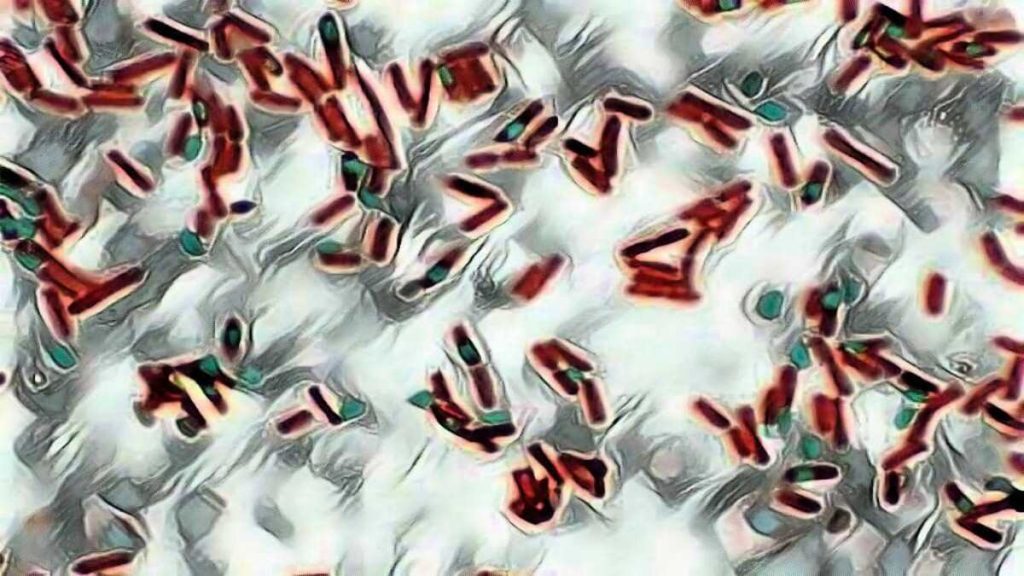
As the endospores are resistant to staining, the endospores are equally resistant to de-staining and will retain the primary dye while the vegetative cells will lose the stain. The addition of a counterstain or secondary stain (safranin) is used to stain the decolorized vegetative cells.
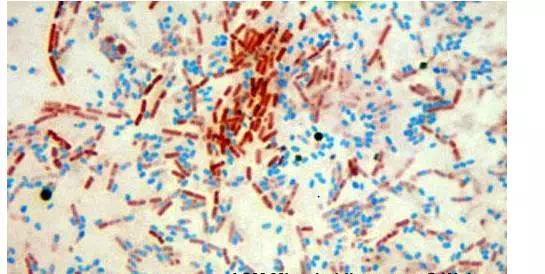
When visualized under microscopy the cells should have three characteristics:
1. the vegetative cells should appear pink/red (i.e. color of counter stain),
2. the vegetative cells that contain endospores should stain pink while the spores should be seen as green ellipses within the cells.
3. Mature, free endospores should not be associated with the vegetative bacteria and should be seen as green ellipses.
Procedure of endospore stain:
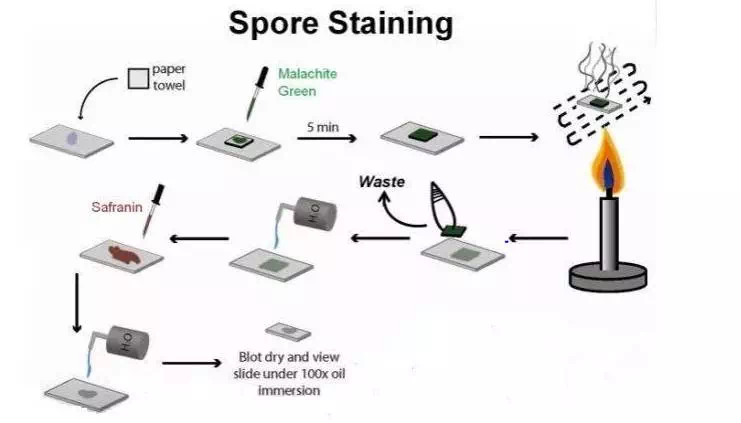
1. Prepare smears of organisms to be tested for presence of endospores on a clean microscope slide and air dry it.
2. Heat fix the smear.
3. Place a small piece of blotting paper (absorbent paper) over the smear and place the slide (smear side up) on a wire gauze on a ring stand.
4. Heat the slide gently till it starts to evaporate (either by putting the slide on a staining rack that has been placed over a boiling water bath or via bunsen burner).
Spore staining procedure
5. Remove the heat and reheat the slide as needed to keep the slide steaming for about 3-5 minutes. As the paper begins to dry add a drop or two of malachite green to keep it moist, but don’t add so much at one time that the temperature is appreciably reduced.
# DO NOT OVERHEAT. The process is steaming and not baking.
6. After 5 minutes carefully remove the slide from the rack using a clothespin
7. Remove the blotting paper and allow the slide to cool to room temperature for 2 minutes.
8. Rinse the slide thoroughly with tap water (to wash the malachite green from both sides of the microscope slide).
9. Stain the smear with safranin for 2 minutes.
10.Rinse both side of the slide to remove the secondary stain and blot the slide/ air dry.

Comments are closed