Sub-protoplasts is divided into three types. The three types are: (1) Mini-protoplasts (2) Cytoplasts and (3) Micro-protoplasts.
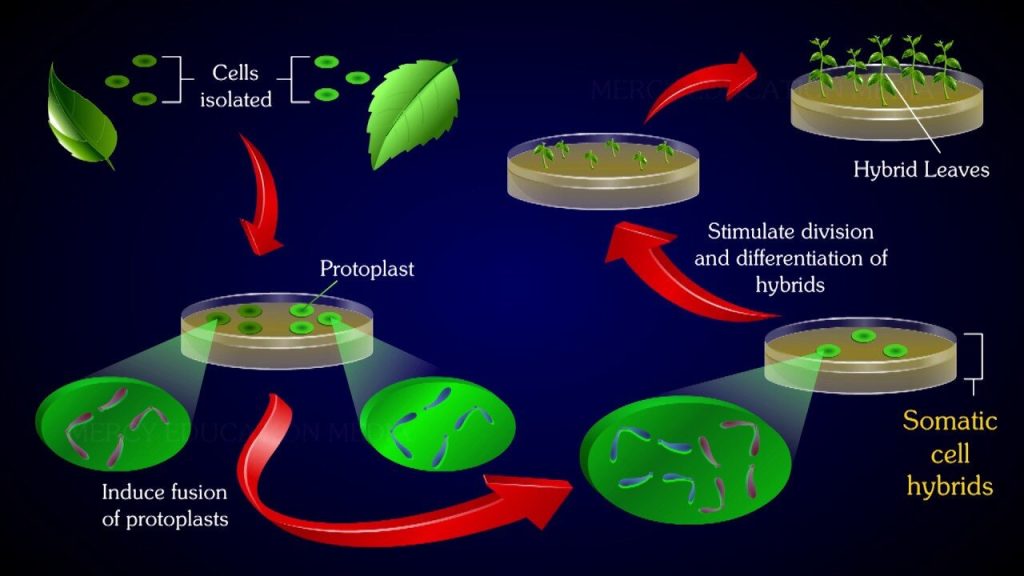
Protoplasts are naked plant cells without the cell wall, but they possess plasma membrane and all other cellular components. They represent the functional plant cells but for the lack of the barrier, cell wall. Protoplasts of different species can be fused to generate a hybrid and this process is referred to as somatic hybridization (or protoplast fusion). Cybridization is the phenomenon of fusion of a normal protoplast with an enucleated (without nucleus) protoplast that results in the formation of a cybrid or cytoplast (cytoplasmic hybrids).
Historical developments:
The term protoplast was introduced in 1880 by Hanstein. The first isolation of protoplasts was achieved by Klercker (1892) employing a mechanical method. A real beginning in protoplast research was made in 1960 by Cocking who used an enzymatic method for the removal of cell wall.
Rakabe and his associates (1971) were successful to achieve the regeneration of whole tobacco plant from protoplasts. Rapid progress occurred after 1980 in protoplast fusion to improve plant genetic material, and the development of transgenic plants.
Importance of Protoplasts and Their Cultures:
The isolation, culture and fusion of protoplasts is a fascinating field in plant research. Protoplast isolation and their cultures provide millions of single cells (comparable to microbial cells) for a variety of studies.
Protoplasts have a wide range of applications; some of them are listed below:
· The protoplast in culture can be regenerated into a whole plant.
· Hybrids can be developed from protoplast fusion.
· It is easy to perform single cell cloning with protoplasts.
· Genetic transformations can be achieved through genetic engineering of protoplast DNA.
· Protoplasts are excellent materials for ultra-structural studies.
· Isolation of cell organelles and chromosomes is easy from protoplasts.
· Protoplasts are useful for membrane studies (transport and uptake processes).
· Isolation of mutants from protoplast cultures is easy.
Isolation of Protoplasts:
Protoplasts are isolated by two techniques
· Mechanical method
· Enzymatic method
Mechanical Method:
Protoplas isolation by mechanical method is a crude and tedious procedure. This results in the isolation of a very small number of protoplasts.
The technique involves the following stages (Fig. 44.1):
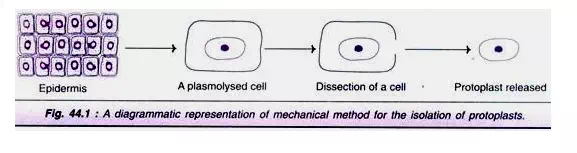
· A small piece of epidermis from a plant is selected.
· The cells are subjected to plasmolysis. This causes protoplasts to shrink away from the cell walls.
· The tissue is dissected to release the protoplasts.
Mechanical method for protoplast isolation is no more in use because of the following limitations:
i. Yield of protoplasts and their viability is low.
ii. It is restricted to certain tissues with vacuolated cells.
iii. The method is laborious and tedious.
However, some workers prefer mechanical methods if the cell wall degrading enzymes (of enzymatic method) cause deleterious effects to protoplasts.
Enzymatic Method:
Enzymatic method is a very widely used technique for the isolation of protoplasts. The advantages of enzymatic method include good yield of viable cells, and minimal or no damage to the protoplasts.
Sources of protoplasts:
Protoplasts can be isolated from a wide variety of tissues and organs that include leaves, roots, shoot apices, fruits, embryos and microspores. Among these, the mesophyll tissue of fully expanded leaves of young plants or new shoots are most frequently used. In addition, callus and suspension cultures also serve as good sources for protoplast isolation.
Enzymes for protoplast isolation:
The enzymes that can digest the cell walls are required for protoplast isolation. Chemically, the plant cell wall is mainly composed of cellulose, hemicellulose and pectin which can be respectively degraded by the enzymes cellulose, hemicellulose and pectinase. The different enzymes for protoplast isolation and the corresponding sources are given in Table 44.1.
In fact, the various enzymes for protoplast isolation are commercially available. The enzymes are usually used at a pH 4.5 to 6.0, temperature 25-30°C with a wide variation in incubation period that may range from half an hour to 20 hours.
The enzymatic isolation of protoplasts can be carried out by two approaches:
1. Two step or sequential method:
The tissue is first treated with pectinase (macerozyme) to separate cells by degrading middle lamella. These free cells are then exposed to cellulose to release protoplasts. Pectinase breaks up the cell aggregates into individual cells while cellulose removes the cell wall proper.
2. One step or simultaneous method:
This is the preferred method for protoplast isolation. It involves the simultaneous use of both the enzymes — macerozyme and cellulose.
Isolation of protoplasts from leaves:
Leaves are most commonly used, for protoplast isolation, since it is possible to isolate uniform cells in large numbers.
The procedure broadly involves the following steps (Fig. 44.2):
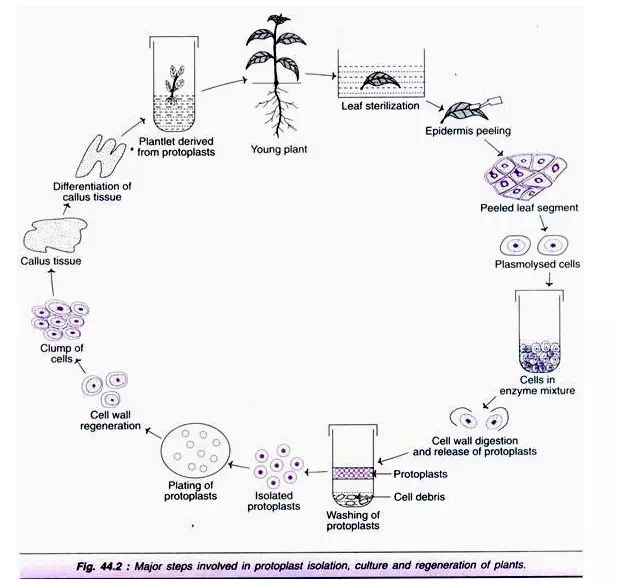
1. Sterilization of leaves.
2. Removal of epidermal cell layer.
3. Treatment with enzymes.
4. Isolation of protoplasts.
Besides leaves, callus cultures and cell suspension cultures can also be used for the isolation of protoplasts. For this purpose, young and actively growing cells are preferred.
Purification of protoplasts:
The enzyme digested plant cells, besides protoplasts contain undigested cells, broken protoplasts and undigested tissues. The cell clumps and undigested tissues can be removed by filtration. This is followed by centrifugation and washings of the protoplasts. After centrifugation, the protoplasts are recovered above Percoll.
Viability of protoplasts:
It is essential to ensure that the isolated protoplasts are healthy and viable so that they are capable of undergoing sustained cell divisions and regeneration.
There are several methods to assess the protoplast viability:
· Fluorescein diacetate (FDA) staining method—The dye accumulates inside viable protoplasts which can be detected by fluorescence microscopy.
· Phenosafranine stain is selectively taken up by dead protoplasts (turn red) while the viable cells remain unstained.
· Exclusion of Evans blue dye by intact membranes.
· Measurement of cell wall formation—Calcofluor white (CFW) stain binds to the newly formed cell walls which emit fluorescence.
· Oxygen uptake by protoplasts can be measured by oxygen electrode.
· Photosynthetic activity of protoplasts.
· The ability of protoplasts to undergo continuous mitotic divisions (this is a direct measure).
Culture of Protoplasts:
The very first step in protoplast culture is the development of a cell wall around the membrane of the protoplast. This is followed by the cell divisions that give rise to a small colony. With suitable manipulations of nutritional and physiological conditions, the cell colonies may be grown continuously as cultures or regenerated to whole plants. Protoplasts are cultured either in semisolid agar or liquid medium. Sometimes, protoplasts are first allowed to develop cell wall in liquid medium, and then transferred to agar medium.
Agar culture:
Agarose is the most frequently used agar to solidify the culture media. The concentration of the agar should be such that it forms a soft agar gel when mixed with the protoplast suspension. The plating of protoplasts is carried out by Bergmann’s cell plating technique .In agar cultures, the protoplasts remain in a fixed position, divide and form cell clones. The advantage with agar culture is that clumping of protoplasts is avoided.
Liquid culture:
Liquid culture is the preferred method for protoplast cultivation for the following reasons:
1. It is easy to dilute and transfer.
2. Density of the cells can be manipulated as desired.
3. For some plant species, the cells cannot divide in agar medium, therefore liquid medium is the only choice.
4. Osmotic pressure of liquid medium can be altered as desired.
Culture Media:
The culture media with regard to nutritional components and osmoticum are briefly described.
Nutritional components:
In general, the nutritional requirements of protoplasts are similar to those of cultured plant cells (callus and suspension cultures). Mostly, MS and B5 media with suitable modifications are used.
Some of the special features of protoplast culture media are listed below:
1. The medium should be devoid of ammonium, and the quantities of iron and zinc should be less.
2. The concentration of calcium should be 2-4-times higher than used for cell cultures. This is needed for membrane stability.
3. High auxin/kinetin ratio is suitable to induce cell divisions while high kinetin/auxin ratio is required for regeneration.
4. Glucose is the preferred carbon source by protoplasts although a combination of sugars (glucose and sucrose) can be used.
5. The vitamins used for protoplast cultures are the same as used in standard tissue culture media.
Osmoticum and osmotic pressure:
Osmoticum broadly refers to the reagents/ chemicals that are added to increase the osmotic pressure of a liquid.
The isolation and culture of protoplasts require osmotic protection until they develop a strong cell wall. In fact, if the freshly isolated protoplasts are directly added to the normal culture medium, they will burst.
Thus, addition of an osmoticum is essential for both isolation and culture media of protoplast to prevent their rupture. The osmotica are of two types — non-ionic and ionic.
Non-ionic osmotica:
The non-ionic substances most commonly used are soluble carbohydrates such as mannitol, sorbitol, glucose, fructose, galactose and sucrose. Mannitol, being metabolically inert, is most frequently used.
Ionic osmotica:
Potassium chloride, calcium chloride and magnesium phosphate are the ionic substances in use to maintain osmotic pressure. When the protoplasts are transferred to a culture medium, the use of metabolically active osmotic stabilizers (e.g., glucose, sucrose) along with metabolically inert osmotic stabilizers (mannitol) is advantageous. As the growth of protoplasts and cell wall regeneration occurs, the metabolically active compounds are utilized, and this results in the reduced osmotic pressure so that proper osmolarity is maintained.
Culture Methods:
The culture techniques of protoplasts are almost the same that are used for cell culture with suitable modifications. Some important aspects are briefly given.
Feeder layer technique:
For culture of protoplasts at low density feeder layer technique is preferred. This method is also important for selection of specific mutant or hybrid cells on plates. The technique consists of exposing protoplast cell suspensions to X-rays (to inhibit cell division with good metabolic activity) and then plating them on agar plates.
Co-culture of protoplasts:
Protoplasts of two different plant species (one slow growing and another fast growing) can be co- cultured. This type of culture is advantageous since the growing species provide the growth factors and other chemicals which help in the generation of cell wall and cell division. The co-culture method is generally used if the two types of protoplasts are morphologically distinct.
Micro drop culture:
Specially designed dishes namely cuprak dishes with outer and inner chambers are used for micro drop culture. The inner chamber carries several wells wherein the individual protoplasts in droplets of nutrient medium can be added. The outer chamber is filled with water to maintain humidity. This method allows the culture of fewer protoplasts for droplet of the medium.
Regeneration of Protoplasts:
Protoplast regeneration which may also be regarded as protoplast development occurs in two stages:
1. Formation of cell wall.
2. Development of callus/whole plant.
Formation of cell wall:
The process of cell wall formation in cultured protoplasts starts within a few hours after isolation that may take two to several days under suitable conditions. As the cell wall development occurs, the protoplasts lose their characteristic spherical shape. The newly developed cell wall by protoplasts can be identified by using calcofluor white fluorescent stain.
The freshly formed cell wall is composed of loosely bound micro fibrils which get organized to form a typical cell wall. This process of cell wall development requires continuous supply of nutrients, particularly a readily metabolised carbon source (e.g. sucrose).
Cell wall development is found to be improper in the presence of ionic osmotic stabilizers in the medium. The protoplasts with proper cell wall development undergo normal cell division. On the other hand, protoplasts with poorly regenerated cell wall show budding and fail to undergo normal mitosis.
Development of Callus/whole Plant:
As the cell wall formation around protoplasts is complete, the cells increase in size, and the first division generally occurs within 2-7 days. Subsequent divisions result in small colonies, and by the end of third week, visible colonies (macroscopic colonies) are formed. These colonies are then transferred to an osmotic-free (mannitol or sorbitol-free) medium for further development to form callus.
With induction and appropriate manipulations, the callus can undergo organogenic or embryo genic differentiation to finally form the whole plant. A general view of the protoplast isolation, culture and regeneration is represented in Fig. 44.2.
Plant regeneration can be done from the callus obtained either from protoplasts or from the culture of plant organs. There are however, certain differences in these two calluses. The callus derived from plant organs carries preformed buds or organized structures, while the callus from protoplast culture does not have such structures.
The first success of regeneration of plants from protoplast cultures of Nicotiana tabacum was achieved by Takebe et al (in 1971). Since then, several species of plants have been regenerated by using protoplasts (Table 44.2).
Sub-Protoplasts:
The fragments derived from protoplasts that do not contain all the contents of plant cells are referred to as sub-protoplasts. It is possible to experimentally induce fragmentation of protoplasts to form sub-protoplasts. This can be done by application of different centrifugal forces created by discontinuous gradients during centrifugation. Exposure of protoplasts to cytochalasin B in association with centrifugation is a better approach for fragmentation of protoplasts.
There are three types of sub-protoplasts (Fig. 44.3):
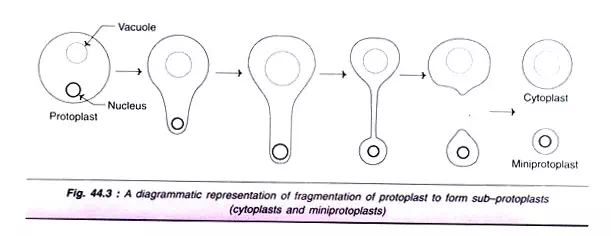
1. Mini-protoplasts:
These are also called as karyoplasts and contain the nucleus. Mini-protoplasts can divide and are capable of regeneration into plants.
2. Cytoplasts:
These are sub-protoplasts containing the original cytoplasmic material (in part or full) but lack nucleus. Thus, cytoplasts are nuclear-free sub-protoplasts which cannot divide, but they can be used for cybridization.
3. Micro-protoplasts:
This term was suggested for sub-protoplasts that contain not all but a few chromosomes.


Comments are closed