Should I make it in liquid form or as a hand bar? Does it need to be extra moisturizing? What intensity of creaminess should the soap contain? These are just a few questions that come to mind when thinking about how useful the saponification process is for making soap. Now, I know you may be thinking, ‘soap,’ as in a Dove bar or a fancy artisan hand soap. Yes, you are thinking along the right lines! This reaction can be used to make soap products ranging from liquid soap to a nice soap bar. But, what is saponification anyway? Great question!
Saponification is an organic chemical reaction that utilizes an alkali to cleave an ester into a carboxylic acid and alcohol. As we will see shortly, the primary use for this reaction is during the production of soap products. The terms ester, carboxylic acid, and alcohol are functional groups. A functional group is simply a group of molecules or atoms that we can easily identify in a compound.
 |
| Functional Groups |
Unfortunately, our friend alkali does not belong to this club. Rather, an alkali is a fancy term for a base that dissolves in solution, producing hydroxide (OH) ions. Given what we know about the background of saponification, let’s discuss the reaction and mechanism in greater detail.
Mechanics: Reaction and Mechanism
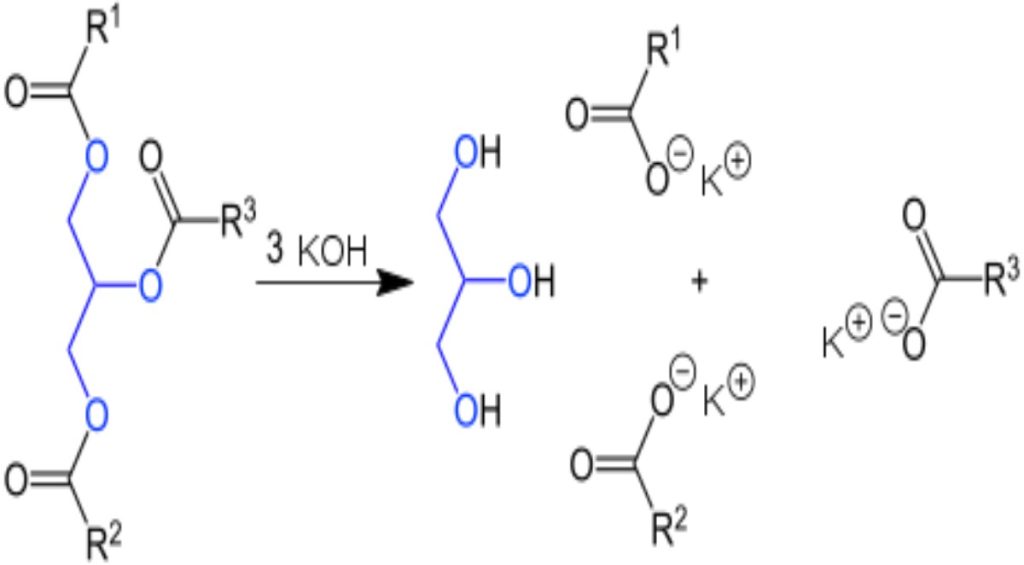
The general saponification reaction is shown on screen here. As you can see, saponification involves two major players: ester and alkali. Note that one of the products is a carboxylate ion. This is simply a carboxylic acid that carries a negative charge once its proton is removed. Use this ion as an ID marker to help you decide whether or not you are dealing with a saponification reaction.
 |
| General Equation for Saponification |
If we look at the mechanism, or instruction guide for this reaction, we will see there is a series of steps that must be followed. Those steps, provided with illustrations, are shown here:
Step 1: The hydroxide ion from the alkali molecule swoops in and performs a nucleophilic attack on the ester or fatty molecule. Don’t be alarmed by the word nucleophile. It simply refers to a molecule that will form a chemical bond due to its attraction to electrons in a different atom or molecule.
 |
| Step 1 of the Saponification Reaction |
Step 2: The OR group becomes a leaving group, following Step 1. OR refers to the oxygen atom bonded to R, which is simply a placeholder for any molecule or atom. Desperately wanting to leave, the OR group departs, causing a double bond to form. This creates a carbonyl group. This new product may look familiar, as it is a carboxylic acid functional group.
 |
| Step 2 of the Saponification Reaction |
Step 3: Not able to leave alone, the OR group plucks the proton (H) from carboxylic acid. This process of removing a proton from a molecule is called deprotonation. Once deprotonated, the final products, carboxylate and alcohol, are formed.
 |
| Step 3 of the Saponification Reaction |
Application: Soap Making
Did you know the root word, ‘sapo-‘ in saponification means soap in Latin? In fact, this name translates to the phrase, ‘soap-making.’ Although there are different uses for the saponification reaction, production of soap is by far the most popular application. If we use a saponification reaction to make soap, we need two substances: a fat and an alkali. The type of fat and alkali greatly influences the end product. Sources of fat can range from your common oils, such as coconut oil or olive oil, to animal fat. The two most common alkalis used are potassium hydroxide (KOH) and sodium hydroxide (NaOH).