Think of Lewis as ‘lectrons’
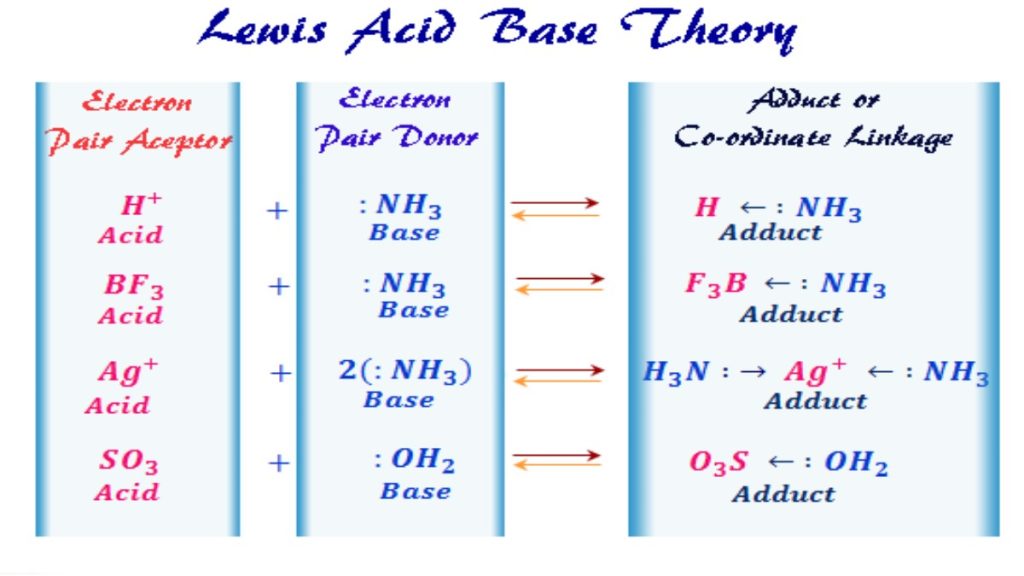
Lewis Acid
A Lewis acid refers to an atom or molecule that accepts an electron pair. Think back to your ‘pushing arrows’ for orgo mechanisms. Every time you draw an arrow representing the movement of electrons, the atom getting attacked or accepting those electrons is the Lewis acid in that reaction.
Common Lewis Acid Examples in Organic Chemistry
§ Borane – BH3 (hydroboration reaction)
§ Aluminum Chloride – AlCl3 (electrophilic aromatic substitution reaction)
§ Iron (III) Bromide – FeBr3 (electrophilic aromatic substitution reaction)
§ and our good friend H+ (keep reading)
The drawing below is part of the EAS Aromatic Halogenation reaction which you’ll see in late Orgo 1 or Orgo 2. Notice how the Fe gets attacked by a lone pair of electrons. By accepting those electrons Fe acts as a Lewis acid

Lewis Base
Since the Lewis definition has to do with the transfer of electrons, you can guess by now that a Lewis Base is an electron pair donor. Once again think back to your reaction mechanisms. The molecule using its electrons to attack another atom is an electron pair donor and a Lewis Base.
Here is the first step in acid catalyzed hydration. The pi bond attacking H+ makes the alkene a Lewis Base.

What If It Fits More Than 1 Category?
After learning the individual acid/base definitions you’re likely confused and overwhelmed.
So much information and so very similar!
And what happens if a molecule appears to fit more than one category?
For example:
Is OH- an Arrhenius Base? Or perhaps its a Bronsted-Lowry or even Lewis Base?
The answer is potentially all 3!
The definitions above evolved slowly as scientists were starting to understand more and more details about chemical reactions. The first is a basic (no pun intended) definition, but the last 2 simply expand to fit more complex solvents and situations.
Take a look at the reaction below where the hydroxide ion attacks a proton on hydronium.

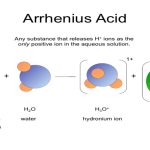
Arrhenius Acid Definition: Hydronium breaks up to yield an H+ in solution.
Arrhenius Base Definition: Hydroxide is an OH- dissolved in water.
Bronsted-Lowry Acid Definition: Hydronium is an H+ donor regardless of solution
Bronsted-Lowry Base Definition: Hydroxide attacks and accepts the H+ from hydronium.
Lewis Acid Definition: The H+ on Hydronium accepts the attacking electron pair to form a bond.
Lewis Base Definition: Hydroxide donates its electron pair to form a bond between itself and H+
Now that you understand the similarities/difference between Arrhenius, Bronsted-Lowry, and Lewis acids and bases, how do you tell which is the stronger or weaker acid? Which molecule is more reactive? Which side of a reaction will be favored at equilibrium?
Learn this and more in my acids and bases tutorial video series:
In conclusion:
As an organic chemistry student you will be required to recognize and classify 3 different types of acids and bases. Arrhenius, Bronsted-Lowry, and Lewis. While the technical definitions vary, once you get the logic behind their definitions you’ll be able to quickly and easily identify the different types of acids and bases.