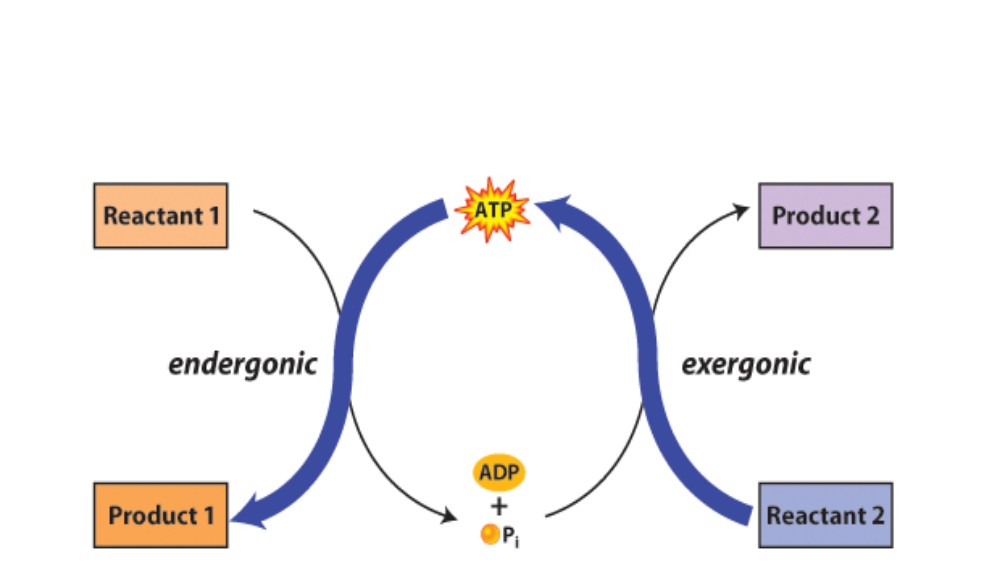
Two or more reactions in a cell sometimes can be coupled so that thermodynamically unfavorable reactions and favorable reactions are combined to drive the overall process in the favorable direction. In this circumstance the overall free energy is the sum of individual free energies of each reaction. This process of coupling reactions is carried out at all levels within cells. The predominant form of coupling is the use of compounds with high energy to drive unfavorable reactions.
The predominant form of high energy compounds in the cell are those which contain phosphate. Hydrolysis of the phosphate group can yield free energies in the range of –2.4 kcal/mol to –14.8kcal/mol (–10 kJ/mol to –62 kJ/mol). The energy of reactions can be given in kcal/mol (the historical standard) or in the International System of Units (SI) standard which is kJoule/mole (kJ/mole). These two different energy values can be interchanged using the conversion factor where 1 kcal/mol = 4.2 kJ/mol. These molecules contain energy in the phosphate bonds due to:
1. Resonance stabilization of the phosphate products
2. Increased hydration of the products
3. Electrostatic repulsion of the products
4. Resonance stabilization of products
5. Proton release in buffered solutions
The latter phenomenon indicates that the pH of the solution a reaction is performed in will influence the equilibrium of the reaction. To account for the fact that all cellular reactions take place in an aqueous environment and that the [H2O] and [H+] are essentially constant these terms in the free energy calculation have been incorporated into a free energy term identified as:

Incorporation of the last equation into a free energy calculation for any reaction in the cell yields:

Examples of Coupled Reactions in Biology
Let’s look at the hypothetical reaction: A → B. If this is a thermodynamically unfavorable reaction the ΔGo’ value will be positive. Let’s assume it is +4.0 kcal/mol. In order to drive this reaction in the direction written it can be coupled to the hydrolysis of ATP. The free energy of ATP hydrolysis to ADP is shown:
ATP + H2O → ADP + Pi: ΔGo’ = –7.3 kcal/mol
Coupling the two reactions together gives the equation:
A + ATP + H2O → B + ADP + Pi + H+
The ΔGo’ for this coupled reaction is the sum of the ΔGo’ values of the two separate reactions, i.e. (–7.3kcal/mol) + (+4.0kcal/mol) = –3.3kcal/mol. This indicates that coupling ATP hydrolysis provides the energy necessary to make the conversion of A to B thermodynamically favorable.
Another useful example is to examine one of the reactions of glycolysis. In this case we will look at the oxidation of phosphoenolpyruvate to pyruvate catalyzed by the enzyme pyruvate kinase (PK).
phosphoenolpyruvate → pyruvate: ΔGo’ = –14.7 kcal/mol
This reaction releases sufficient energy to drive the synthesis of ATP from ADP and Pi which would normally be thermodynamically unfavorable with a ΔGo’ of +7.3kcal/mol. Note that this value is the reciprocal of the hydrolysis of ATP. This points out another fact that the ΔGo’ for a reaction in one direction is equal but mathematically opposite for the reciprocal direction. Coupling the two reactions together yields:
phosphoenolpyruvate + ADP + H+ → pyruvate + ATP: ΔGo’ = –7.4 kcal/mol