The adaptive, or acquired, immune response takes days or even weeks to become established—much longer than the innate response; however, adaptive immunity is more specific to an invading pathogen. Adaptive immunity is an immunity that occurs after exposure to an antigen either from a pathogen or a vaccination. An antigen is a molecule that stimulates a response in the immune system. This part of the immune system is activated when the innate immune response is insufficient to control an infection. In fact, without information from the innate immune system, the adaptive response could not be mobilized. There are two types of adaptive responses: the cell-mediated immune response, which is controlled by activated T cells, and the humoral immune response, which is controlled by activated B cells and antibodies. Activated T and B cells whose surface binding sites are specific to the molecules on the pathogen greatly increase in numbers and attack the invading pathogen. Their attack can kill pathogens directly or they can secrete antibodies that enhance the phagocytosis of pathogens and disrupt the infection. Adaptive immunity also involves a memory to give the host long-term protection from reinfection with the same type of pathogen; on reexposure, this host memory will facilitate a rapid and powerful response.
B and T Cells
Lymphocytes, which are white blood cells, are formed with other blood cells in the red bone marrow found in many flat bones, such as the shoulder or pelvic bones. The two types of lymphocytes of the adaptive immune response are B and T cells (Figure 12.12). Whether an immature lymphocyte becomes a B cell or T cell depends on where in the body it matures. The B cells remain in the bone marrow to mature (hence the name “B” for “bone marrow”), while T cells migrate to the thymus, where they mature (hence the name “T” for “thymus”).
Maturation of a B or T cell involves becoming immunocompetent, meaning that it can recognize, by binding, a specific molecule or antigen (discussed below). During the maturation process, B and T cells that bind too strongly to the body’s own cells are eliminated in order to minimize an immune response against the body’s own tissues. Those cells that react weakly to the body’s own cells, but have highly specific receptors on their cell surfaces that allow them to recognize a foreign molecule, or antigen, remain. This process occurs during fetal development and continues throughout life. The specificity of this receptor is determined by the genetics of the individual and is present before a foreign molecule is introduced to the body or encountered. Thus, it is genetics and not experience that initially provides a vast array of cells, each capable of binding to a different specific foreign molecule. Once they are immunocompetent, the T and B cells will migrate to the spleen and lymph nodes where they will remain until they are called on during an infection. B cells are involved in the humoral immune response, which targets pathogens loose in blood and lymph, and T cells are involved in the cell-mediated immune response, which targets infected cells.

Figure 12.12: This scanning electron micrograph shows a T lymphocyte. T and B cells are indistinguishable by light microscopy but can be differentiated experimentally by probing their surface receptors. (credit: modification of work by NCI; scale-bar data from Matt Russell)
Humoral Immune Response
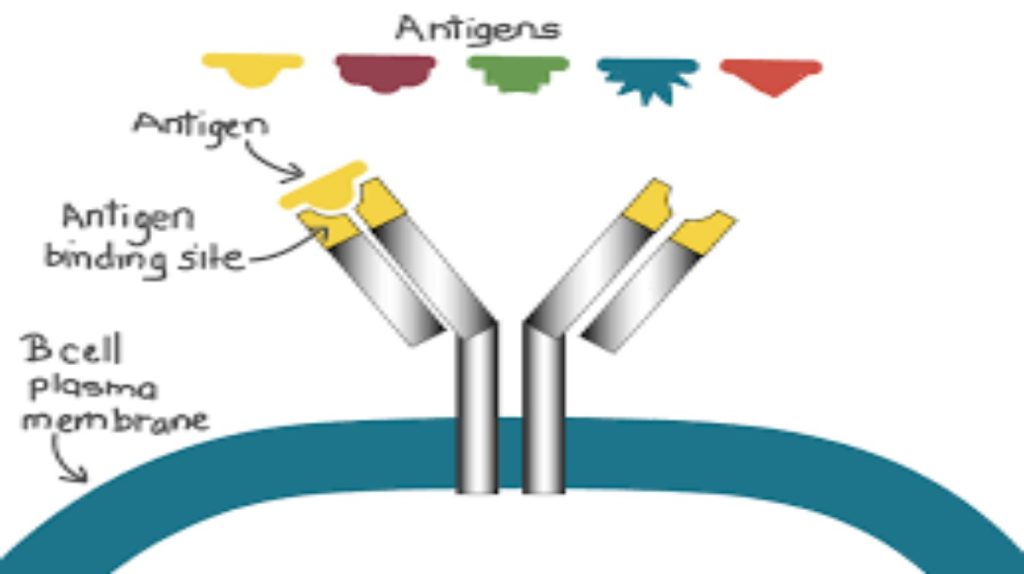
As mentioned, an antigen is a molecule that stimulates a response in the immune system. Not every molecule is antigenic. B cells participate in a chemical response to antigens present in the body by producing specific antibodies that circulate throughout the body and bind with the antigen whenever it is encountered. This is known as the humoral immune response. As discussed, during maturation of B cells, a set of highly specific B cells are produced that have many antigen receptor molecules in their membrane (Figure 12.13).
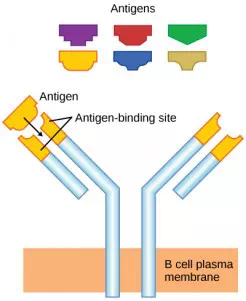
Figure 12.13. B cell receptors are embedded in the membranes of B cells and bind a variety of antigens through their variable regions.
Each B cell has only one kind of antigen receptor, which makes every B cell different. Once the B cells mature in the bone marrow, they migrate to lymph nodes or other lymphatic organs. When a B cell encounters the antigen that binds to its receptor, the antigen molecule is brought into the cell by endocytosis and reappears on the surface of the cell bound to an MHC class II molecule. When this process is complete, the B cell is sensitized. In most cases, the sensitized B cell must then encounter a specific kind of T cell, called a helper T cell, before it is activated. The helper T cell must already have been activated through an encounter with the antigen (discussed below).
The helper T cell binds to the antigen-MHC class II complex and is induced to release cytokines that induce the B cell to divide rapidly, which makes thousands of identical (clonal) cells. These daughter cells become either plasma cells or memory B cells. The memory B cells remain inactive at this point, until another later encounter with the antigen, caused by a reinfection by the same bacteria or virus, results in them dividing into a new population of plasma cells. The plasma cells, on the other hand, produce and secrete large quantities, up to 100 million molecules per hour, of antibody molecules. An antibody, also known as an immunoglobulin (Ig), is a protein that is produced by plasma cells after stimulation by an antigen. Antibodies are the agents of humoral immunity. Antibodies occur in the blood, in gastric and mucus secretions, and in breast milk. Antibodies in these bodily fluids can bind pathogens and mark them for destruction by phagocytes before they can infect cells.
These antibodies circulate in the blood stream and lymphatic system and bind with the antigen whenever it is encountered. The binding can fight infection in several ways. Antibodies can bind to viruses or bacteria and interfere with the chemical interactions required for them to infect or bind to other cells. The antibodies may create bridges between different particles containing antigenic sites clumping them all together and preventing their proper functioning. The antigen-antibody complex stimulates the complement system described previously, destroying the cell bearing the antigen. Phagocytic cells, such as those already described, are attracted by the antigen-antibody complexes, and phagocytosis is enhanced when the complexes are present. Finally, antibodies stimulate inflammation, and their presence in mucus and on the skin prevents pathogen attack.
Antibodies coat extracellular pathogens and neutralize them by blocking key sites on the pathogen that enhance their infectivity (such as receptors that “dock” pathogens on host cells) (Figure 12.14). Antibody neutralization can prevent pathogens from entering and infecting host cells. The neutralized antibody-coated pathogens can then be filtered by the spleen and eliminated in urine or feces.
Antibodies also mark pathogens for destruction by phagocytic cells, such as macrophages or neutrophils, in a process called opsonization. In a process called complement fixation, some antibodies provide a place for complement proteins to bind. The combination of antibodies and complement promotes rapid clearing of pathogens.
The production of antibodies by plasma cells in response to an antigen is called active immunityand describes the host’s active response of the immune system to an infection or to a vaccination. There is also a passive immune response where antibodies come from an outside source, instead of the individual’s own plasma cells, and are introduced into the host. For example, antibodies circulating in a pregnant woman’s body move across the placenta into the developing fetus. The child benefits from the presence of these antibodies for up to several months after birth. In addition, a passive immune response is possible by injecting antibodies into an individual in the form of an antivenom to a snake-bite toxin or antibodies in blood serum to help fight a hepatitis infection. This gives immediate protection since the body does not need the time required to mount its own response.
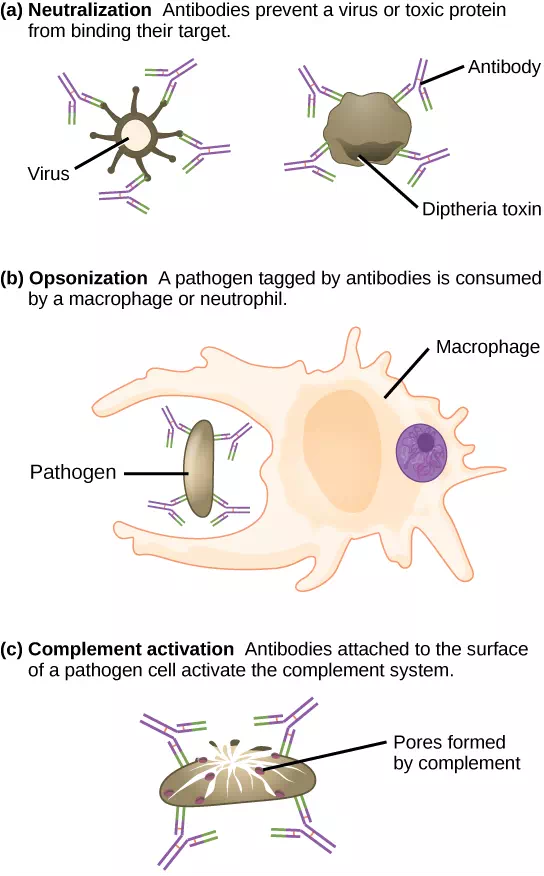
Figure 12.14 Antibodies may inhibit infection by (a) preventing the antigen from binding its target, (b) tagging a pathogen for destruction by macrophages or neutrophils, or (c) activating the complement cascade.
Comments are closed