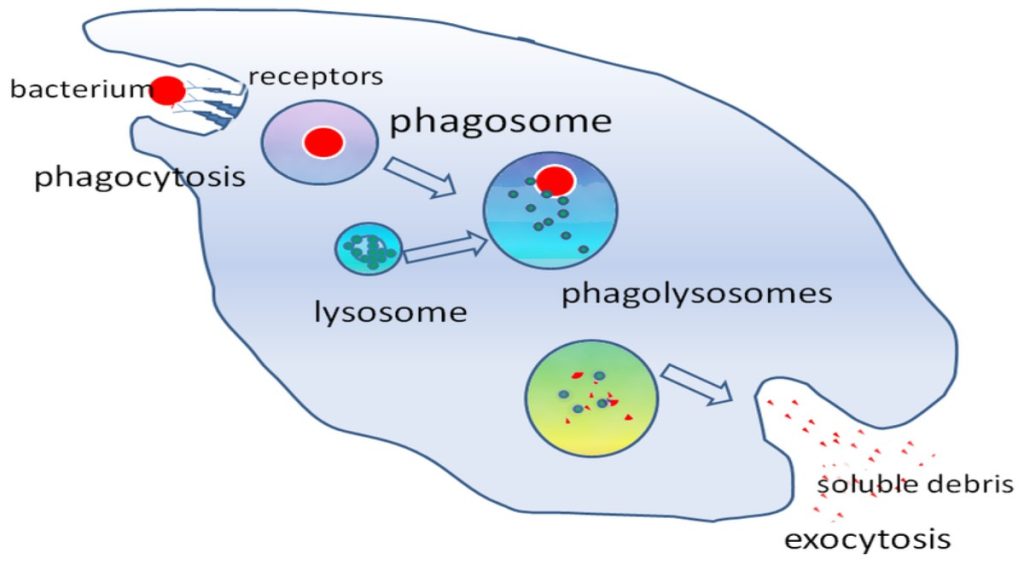
The first cytokines to be produced are pro-inflammatory; that is, they encourage inflammation, the localized redness, swelling, heat, and pain that result from the movement of leukocytes and fluid through increasingly permeable capillaries to a site of infection. The population of leukocytes that arrives at an infection site depends on the nature of the infecting pathogen. Both macrophages and dendritic cells engulf pathogens and cellular debris through phagocytosis. A neutrophil is also a phagocytic leukocyte that engulfs and digests pathogens. Neutrophils, shown in Figure 23.3, are the most abundant leukocytes of the immune system. Neutrophils have a nucleus with two to five lobes, and they contain organelles, called lysosomes, that digest engulfed pathogens. An eosinophilis a leukocyte that works with other eosinophils to surround a parasite; it is involved in the allergic response and in protection against helminthes (parasitic worms).
Neutrophils and eosinophils are particularly important leukocytes that engulf large pathogens, such as bacteria and fungi. A mast cell is a leukocyte that produces inflammatory molecules, such as histamine, in response to large pathogens. A basophil is a leukocyte that, like a neutrophil, releases chemicals to stimulate the inflammatory response as illustrated in Figure 23.5. Basophils are also involved in allergy and hypersensitivity responses and induce specific types of inflammatory responses. Eosinophils and basophils produce additional inflammatory mediators to recruit more leukocytes. A hypersensitive immune response to harmless antigens, such as in pollen, often involves the release of histamine by basophils and mast cells.
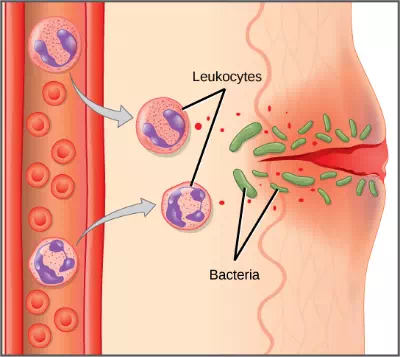
Figure 23.5. In response to a cut, mast cells secrete histamines that cause nearby capillaries to dilate. Neutrophils and monocytes leave the capillaries. Monocytes mature into macrophages. Neutrophils, dendritic cells and macrophages release chemicals to stimulate the inflammatory response. Neutrophils and macrophages also consume invading bacteria by phagocytosis.
Cytokines also send feedback to cells of the nervous system to bring about the overall symptoms of feeling sick, which include lethargy, muscle pain, and nausea. These effects may have evolved because the symptoms encourage the individual to rest and prevent them from spreading the infection to others. Cytokines also increase the core body temperature, causing a fever, which causes the liver to withhold iron from the blood. Without iron, certain pathogens, such as some bacteria, are unable to replicate; this is called nutritional immunity.
Natural Killer Cells
Lymphocytes are leukocytes that are histologically identifiable by their large, darkly staining nuclei; they are small cells with very little cytoplasm, as shown in Figure 23.6. Infected cells are identified and destroyed by natural killer (NK) cells, lymphocytes that can kill cells infected with viruses or tumor cells (abnormal cells that uncontrollably divide and invade other tissue). T cells and B cells of the adaptive immune system also are classified as lymphocytes. T cells are lymphocytes that mature in the thymus gland, and B cells are lymphocytes that mature in the bone marrow. NK cells identify intracellular infections, especially from viruses, by the altered expression of major histocompatibility class (MHC) I molecules on the surface of infected cells. MHC I molecules are proteins on the surfaces of all nucleated cells, thus they are scarce on red blood cells and platelets which are non-nucleated. The function of MHC I molecules is to display fragments of proteins from the infectious agents within the cell to T-cells; healthy cells will be ignored, while “non-self” or foreign proteins will be attacked by the immune system. MHC II molecules are found mainly on cells containing antigens (“non-self proteins”) and on lymphocytes. MHC II moleculesinteract with helper T-cells to trigger the appropriate immune response, which may include the inflammatory response.
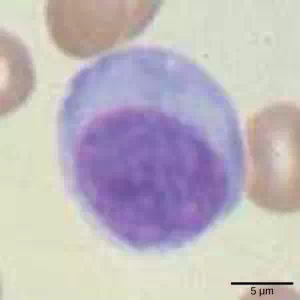
Figure 23.6. Lymphocytes, such as NK cells, are characterized by their large nuclei that actively absorb Wright stain and therefore appear dark colored under a microscope.
An infected cell (or a tumor cell) is usually incapable of synthesizing and displaying MHC I molecules appropriately. The metabolic resources of cells infected by some viruses produce proteins that interfere with MHC I processing and/or trafficking to the cell surface. The reduced MHC I on host cells varies from virus to virus and results from active inhibitors being produced by the viruses. This process can deplete host MHC I molecules on the cell surface, which NK cells detect as “unhealthy” or “abnormal” while searching for cellular MHC I molecules. Similarly, the dramatically altered gene expression of tumor cells leads to expression of extremely deformed or absent MHC I molecules that also signal “unhealthy” or “abnormal.”
NK cells are always active; an interaction with normal, intact MHC I molecules on a healthy cell disables the killing sequence, and the NK cell moves on. After the NK cell detects an infected or tumor cell, its cytoplasm secretes granules comprised of perforin, a destructive protein that creates a pore in the target cell. Granzymes are released along with the perforin in the immunological synapse. A granzyme is a protease that digests cellular proteins and induces the target cell to undergo programmed cell death, or apoptosis. Phagocytic cells then digest the cell debris left behind. NK cells are constantly patrolling the body and are an effective mechanism for controlling potential infections and preventing cancer progression.
Complement
An array of approximately 20 types of soluble proteins, called a complement system, functions to destroy extracellular pathogens. Cells of the liver and macrophages synthesize complement proteins continuously; these proteins are abundant in the blood serum and are capable of responding immediately to infecting microorganisms. The complement system is so named because it is complementary to the antibody response of the adaptive immune system. Complement proteins bind to the surfaces of microorganisms and are particularly attracted to pathogens that are already bound by antibodies. Binding of complement proteins occurs in a specific and highly regulated sequence, with each successive protein being activated by cleavage and/or structural changes induced upon binding of the preceding protein(s). After the first few complement proteins bind, a cascade of sequential binding events follows in which the pathogen rapidly becomes coated in complement proteins.
Complement proteins perform several functions. The proteins serve as a marker to indicate the presence of a pathogen to phagocytic cells, such as macrophages and B cells, and enhance engulfment; this process is called opsonization. Certain complement proteins can combine to form attack complexes that open pores in microbial cell membranes. These structures destroy pathogens by causing their contents to leak, as illustrated in Figure 23.7.
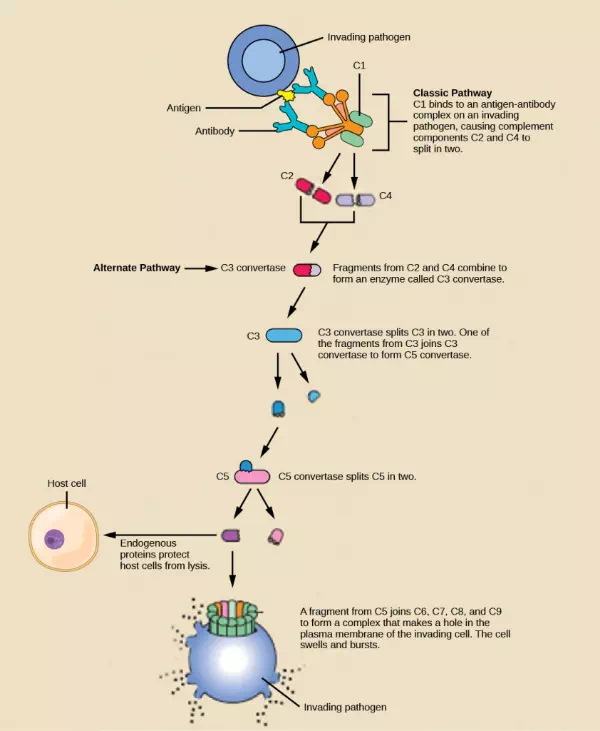
Figure 23.7. The classic pathway for the complement cascade involves the attachment of several initial complement proteins to an antibody-bound pathogen followed by rapid activation and binding of many more complement proteins and the creation of destructive pores in the microbial cell envelope and cell wall. The alternate pathway does not involve antibody activation. Rather, C3 convertase spontaneously breaks down C3. Endogenous regulatory proteins prevent the complement complex from binding to host cells. Pathogens lacking these regulatory proteins are lysed. (credit: modification of work by NIH)
Summary
The innate immune system serves as a first responder to pathogenic threats that bypass natural physical and chemical barriers of the body. Using a combination of cellular and molecular attacks, the innate immune system identifies the nature of a pathogen and responds with inflammation, phagocytosis, cytokine release, destruction by NK cells, and/or a complement system. When innate mechanisms are insufficient to clear an infection, the adaptive immune response is informed and mobilized.